The immune reconstitution (IR) after the allogenic hematopoietic stem cell transplantation (allo-HSCT) is a progressive process intrinsically correlated to the therapeutic success. It is essential to understand the interfering factors in IR to prevent the HSCT-related mortality.
MethodsWe retrospectively evaluated the clinical outcomes, absolute lymphocyte counts (ALCs) and lymphocyte subtypes at different time-points of 111 pediatric patients with allogeneic HSCT for malignant and non-malignant diseases from 2013 to 2018.
ResultsThe ALCs gradually increased on D+30, D+100, and D+180 (medians 634/μL, 1022/μL and 1541/μL, respectively). On D+100, the CD3+CD8+ achieved the highest recovery rate (68%), followed by the CD16+CD56+ (47%), CD3+CD4+ (39%) and CD19+ (8%). The adequate ALC recovery was associated with age < 8 years, bone marrow grafts, myeloablative conditioning, non-use of serotherapy and non-haploidentical donors. The ALC and CD3+CD8+ on D+100 counts were higher in patients with the cytomegalovirus infection. The CD3+CD4+ recovery was associated with an age < 8 years, a non-malignant disease and a lower incidence of acute graft-versus-host disease ≥ grade 2. Furthermore, the ALC recovery on D+100 resulted in a higher overall survival, regardless of the disease type (HR 3.65, 1.05 - 12.71, p = 0.04).
ConclusionSeveral factors influenced the IR after the allo-HSCT. The ALC ≥ 500/μL on D+100 was a simple IR predictor of survival, easily available to resource-limited centers.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potential treatment for many patients with malignant and non-malignant diseases. Although much improvement has been made during the past decades, several complications affecting patient survival or quality of life remain to be addressed.1
It is well established that immune reconstitution (IR) after the HSCT is influenced by patient and transplant factors and strongly associated with transplant outcomes.2 The disease type, recipient age, source of stem cells, type of donor, conditioning regimen, use of serotherapy, prophylaxis and treatment for graft-versus-host disease (GvHD) and viral infections may have a significant impact on the IR. Despite numerous studies, the predictive immune parameters of clinical outcomes remain elusive due to the variety of cut-off values for lymphocyte counts, the time of the post-HSCT for blood sampling and the group of patients included in each study.3
There is a dearth of studies regarding the IR after the pediatric HSCT, especially for patients transplanted in resource-limited countries. In many places around the world, the access to this curative procedure is hindered by socioeconomic problems, the unavailability of well-matched donors and the lack of drugs and tests. These barriers culminate in a lower volume of HSCT and, consequently, in the lower number of publications on this topic, compared to North America and Europe.4
Our objective was to analyze the absolute lymphocyte count (ALC) and lymphocyte subpopulations after the allo-HSCT and correlate these factors with transplant characteristics and major post-transplant outcomes.
MethodsThis study was conducted at the HSCT Unit of the Hospital Pequeno Príncipe in Curitiba, one of Brazil's largest pediatric HSCT centers. Financed mainly by public resources, the center has been performing autologous HSCTs since 2011. Subsequently, the allo-HSCT was established for matched sibling donors (MSDs) (2013), haploidentical donors (2014) and unrelated donors (2017). This is a retrospective study based on descriptive and quantitative analyses. The data were obtained from the hospital database with the prior local Ethics Committee approval (3.318.929).
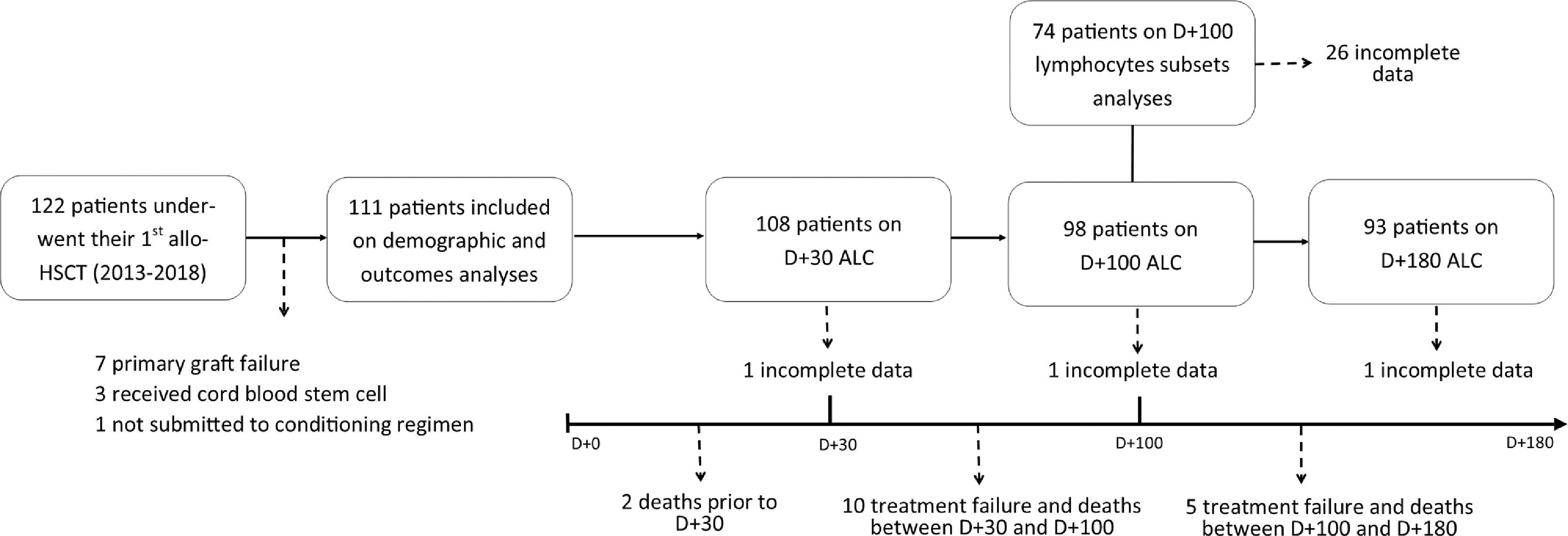
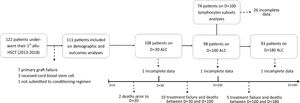
Patient cohortFrom January 2013 to December 2018, 122 patients under 18 years of age underwent their first allo-HSCT. From this initial group, those who experienced primary graft failure (n = 7), received stem cells from umbilical cord blood (n = 3) or did not undergo a conditioning regimen (n = 1) were excluded. The final cohort included 111 patients. For lymphocyte recovery analysis, patients who experienced treatment failure (secondary graft failure or relapse), those who died before each cut-off day and those with incomplete medical records were also excluded (Figure 1).
Conditioning regimen and GvHD immunoprophylaxisConditioning regimens consisted of cyclophosphamide + total body irradiation (CY+TBI, n = 28), busulfan + fludarabine ± rabbit antithymocyte globulin/alemtuzumab (BU+FLU±ATG/Alemtuzumab, n = 22), CY±ATG (n = 14), FLU+TBI (n = 12), BU+CY (n = 10), CY+FLU+TBI±ATG (n = 8), CY+FLU±ATG (n = 6), BU+FLU + melphalan ± ATG (n=5) and other regimens (n = 6). A total of 36 patients received serotherapy in the conditioning regimen (ATG, n = 35; Alemtuzumab, n = 1). The GVHD prophylaxis was based on cyclosporine + methotrexate (CsA+MTX, n = 62, 56%), post-transplant cyclophosphamide + cyclosporine + mycophenolate mofetil (PT-Cy+CsA+MMF, n = 31, 28%), CsA+MMF (n = 12, 11%) and others (n = 6, 5%).
DefinitionsThe date of the stem cell infusion was defined as day zero. The lymphocyte reconstitution refers to the quantitative count of cells, while the lymphocyte recovery refers to the reconstitution categorization. The lymphocyte recovery was classified based on the ALC in peripheral blood obtained, using the complete blood count routinely performed on D+30, D+100 and D+180. Previous studies defined the lymphocyte recovery as ALC ≥ 300/μL for D+30, ALC ≥ 500/μL for D+100 and ALC ≥ 750/μL for D+180.5,6
The flow cytometry immunophenotyping of the peripheral blood was used to assess CD3+CD4+, CD3+CD8+, CD16+CD56+ and CD19+ counts obtained 100 days after the HSCT (± 14 days). The recovery of subtypes CD3+CD4+, CD3+CD8+ and CD19+ were classified as absolute counts ≥ 200/μL and ≥ 150/μL for CD16+CD56+. Thus, patients with absolute counts below the described values were classified as not recovered, based on previous studies.7
The neutrophil and platelet counts confirmed the engraftment in peripheral blood. The neutrophil engraftment was considered the first day of three consecutive days with the neutrophil count ≥ 500/μL and the platelet engraftment was the seventh day with the platelet count ≥ 20,000/μL unsupported by transfusion.8 The cytomegalovirus (CMV) surveillance was screened using the pp65 antigenemia or DNA detection by the quantitative PCR assay. The CMV reactivation and infection (in case of negative serostatus pre-HSCT) were defined by the antigen or DNA detection in the peripheral blood. The term CMV infection refers to both reactivation and infection. Acute GvHD was defined using the standard clinical and laboratory criteria and classified as grades 1 to 4, considering only acute graft-versus-host disease (aGvHD) ≥ grade 2 as clinically significant.9 The chronic GvHD was not analyzed due to the reduced number of cases up to D+180. Among hematological malignancies, the disease phase was defined as early, in the case of the first or second remission, and advanced, in the case of the third or fourth remission, or active disease at the time of the HSCT.
StatisticsWe used the Chi-square or Fisher's exact test to analyze the association of lymphocyte recovery with the following variables: gender, age group (≥ and < 8 years, [median age]), disease type (malignant, non-malignant), disease phase (early, advanced), donor type (MSD, haploidentical, unrelated), ABO compatibility (compatible, incompatible), stem cell source (peripheral blood stem cells [PBSC], bone marrow [BM]), conditioning regimen (myeloablative conditioning [MAC], reduced-intensity conditioning [RIC]) and use of serotherapy (yes, no). The Mann-Whitney and Kruskal-Wallis tests were used for continuous variables due to the lack of normal distribution.
Time-dependent variables were calculated from the date of the HSCT to the event. Cumulative incidences (CIs) were estimated using the Gray's method, accounting for competing risks. Death without an event was considered a competing risk for all variables, however, it was also included for the aGvHD graft failure. To evaluate the effect of significant variables on such outcomes, we performed the Fine-Gray's competing risks univariate and multivariate regression. The overall survival (OS) was analyzed using the Kaplan-Meier method and differences between groups were estimated using the log-rank test. Univariate and multivariate Cox regression models were used to assess the impact of the significant variables. It was not possible to evaluate outcomes for subgroups of immune recovery with a small sample size. All p-values < 0.05 were considered statistically significant. Statistical analyses were performed using the SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and the EZR version 1.40 (Saitama Medical Center, Jichi Medical University, Japan).
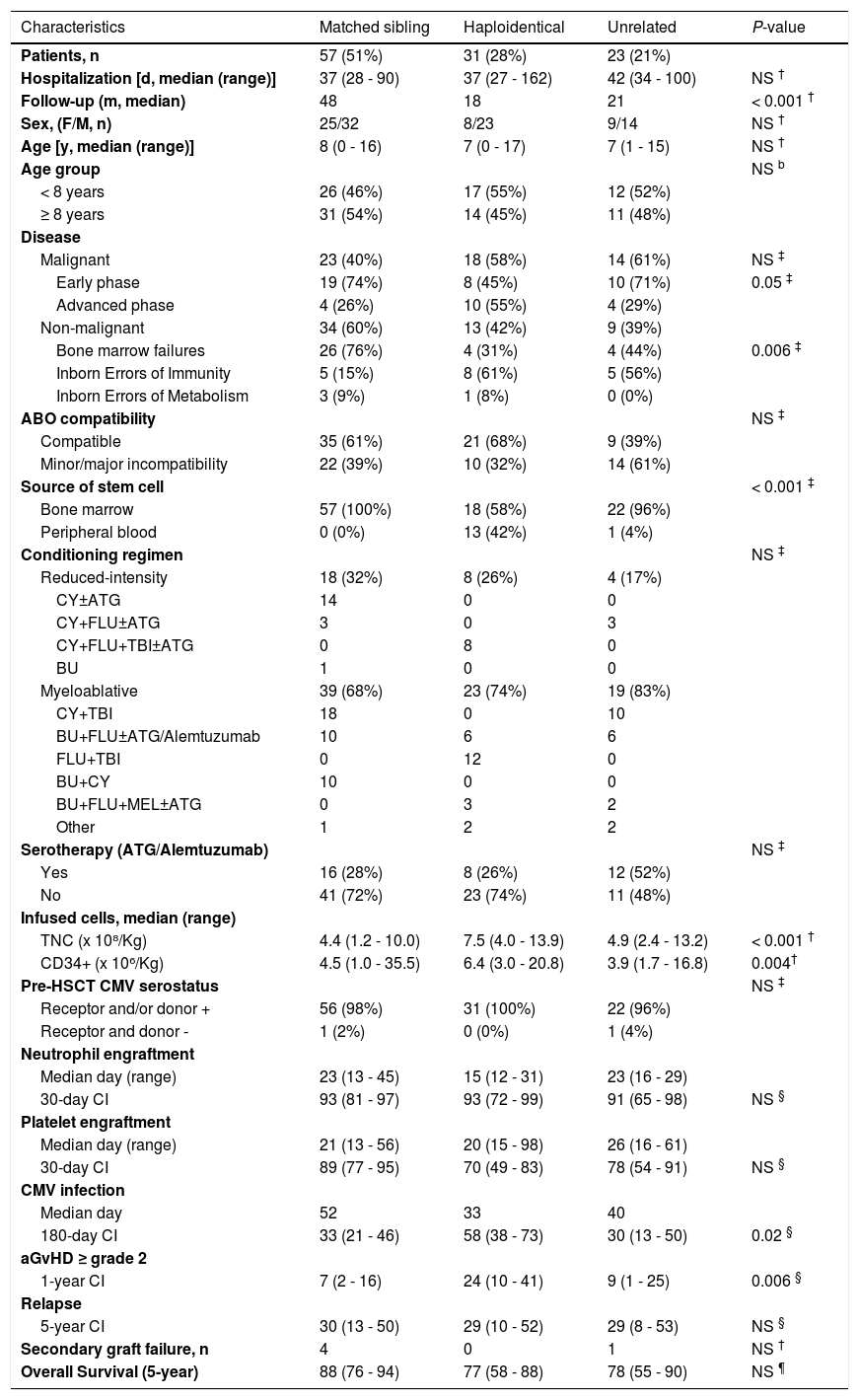
ResultsPatient characteristics and outcomes are described in Table 1. Most patients (59%) were from other Brazilian states, spanning up to 3,400 kilometers from our unit.
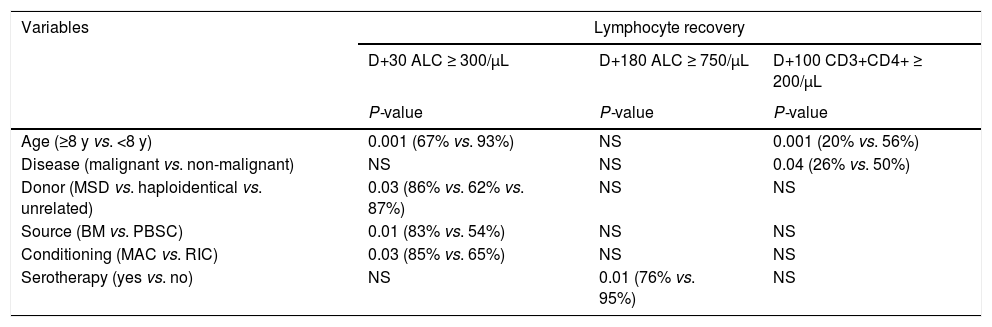
Patient characteristics and outcomes according to the donor.
Data in parentheses represent the respective percentage, unless specified. Other myeloablative regimens include: BU+CY+MEL, BU+FLU+ Thiotepa, CY+TBI+ATG, FLU+BU+CY and FLU+CY+MEL. Statistical analyses were performed using Kruskal-Wallis test for continuous data (†), Chi-square or Fisher test for categorical data (‡), Gray's test for time-dependent variables (§) and Kaplan-Meier for overall survival (¶). Abbreviations: aGvHD, acute graft-versus-host disease; ATG, antithymocyte globulin; BU, busulfan; CI, Cumulative incidence (95% confidence interval); CMV, cytomegalovirus; CY, cyclophosphamide; d, days; F, female; FLU, fludarabine; HSCT, hematopoietic stem cell transplantation; MEL, melphalan; m, months; M, male; n, number; NS, not significant; TBI, total body irradiation; TNC, total nucleated cells; y, years.
The cohort consisted of malignant and non-malignant diseases (BM failure syndromes, inborn errors of immunity and inborn errors of metabolism). Diagnoses of malignant diseases were acute lymphoblastic leukemia (n = 37), acute myeloblastic leukemia (n = 10), acute biphenotypic leukemia (n = 4), myelodysplastic syndrome (n = 2), juvenile myelomonocytic leukemia (n = 1) and non-Hodgkin's lymphoma (n = 1). Bone marrow failure syndromes were acquired severe aplastic anemia (n = 14), Fanconi anemia (n = 11), Diamond-Blackfan anemia (n = 7) and dyskeratosis congenita (n = 2). Inborn errors of immunity included severe combined immunodeficiency (n = 9), Wiskott Aldrich syndrome (n = 5), hemophagocytic lymphohistiocytosis (n = 2), CD40L deficiency (n = 1) and Griscelli syndrome (n = 1). The adrenoleukodystrophy (n = 3) and mucopolysaccharidosis type I (n = 1) represented the inborn errors of the metabolism.
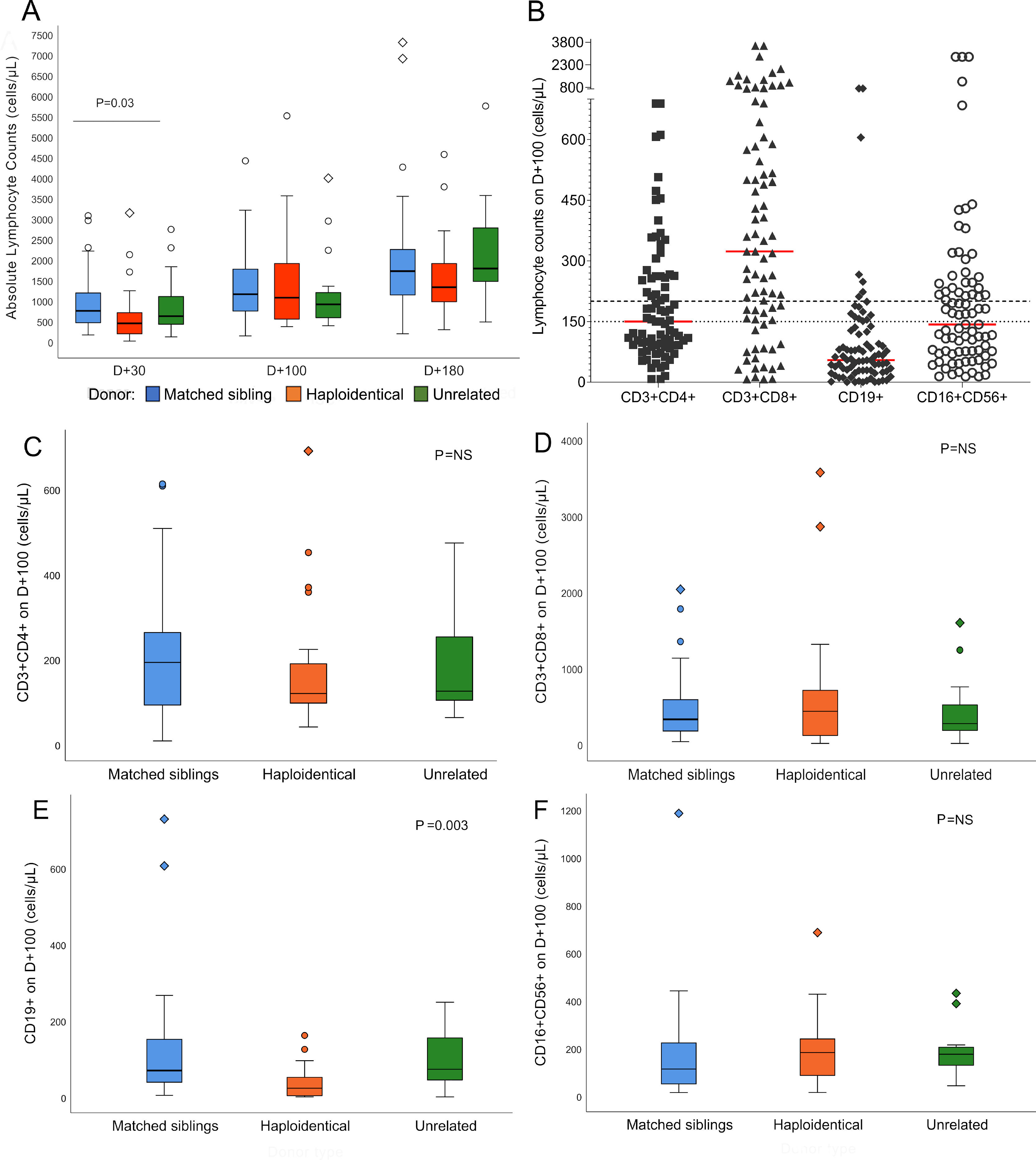
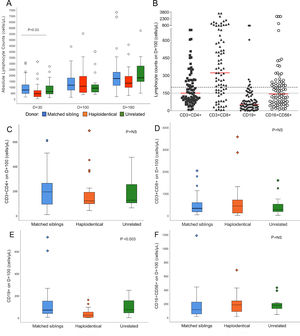
Lymphocyte reconstitutionSerial ALC assessments showed a gradual increase from D+30 to D+180. Median counts reached 634/µL (8 – 3,133) on D+30, 1,022/µL (133 – 5,503) on D+100 and 1,541/µL (187 – 14,432) on D+180, representing 82%, 89% and 90% of recovered patients, respectively. The difference in the ALC according to the donor was significant (p = 0.03) on D+30 (Figure 2A).
Lymphocyte reconstitution after hematopoietic stem cell transplantation. A: Absolute lymphocyte counts on D+30, D+100 and D+180 according to the type of donor. Median absolute lymphocyte count on D+30: matched siblings 746/µL, haploidentical 441/µL and unrelated 616/µL, p = 0.03; D+100: matched siblings 1,152/µL, haploidentical 1,066/µL and unrelated 903/µL, p = not significant; D+180: matched siblings 1,714/µL, haploidentical 1,321/µL and unrelated 1,778/µL, p = not significant. The line over bars indicates groups significantly different (Kruskal-Wallis test). For scale reasons, three outlier counts on D+180 are not shown (10,195/µL of matched siblings, 10,404/µL of haploidentical and 14,432/µL of unrelated). B: Lymphocyte subtypes counts on D+100. Red lines represent the medians for each subtype. Dashed line shows the 200/µL cut-off value for CD3+CD4+, CD3+CD8+ and CD19+. The dotted line shows the 150/µL cut-off value for CD16+CD56+. C-F: Reconstitution of lymphocyte subpopulations on D+100 according to the type of donor (n = 74, Kruskal-Wallis test). C: CD3+CD4+ reconstitution. D: CD3+CD8+ reconstitution. E: CD19+ reconstitution. F: CD16+CD56+ reconstitution. Abbreviations: D+30, day+30; D+100, day +100; D+180, day +180; NS, not significant. In boxplot graphs, circles (○) represent outlier values and diamonds (◇) represent far outlier values.
The flow cytometry analysis on D+100 showed the highest CD3+CD8+ median count (336/µL, 8 – 3,569), followed by CD3+CD4+ (154/µL, 8 - 689), CD16+CD56+ (130/µL, 14 – 1,184) and CD19+ (56/µL, 0 - 727), representing 68%, 34%, 47% and 8% of patients above the cut-off values, respectively. The CD19+ reconstitution was slow and varied significantly according to the donor type. On D+100, the haploidentical HSCT (haplo-HSCT) had a median of 23/µL, whereas the MSD was 69/µL and the unrelated HSCT was 72/µL, p = 0.003 (Figure 2E).
Factors associated with lymphocyte recoveryVariables associated with the ALC and lymphocyte subpopulation recoveries are described in Table 2. No association was found with the sex, status of malignancy, ABO compatibility or dose of the total nucleated cells (TNCs) and CD34+ infused. The CD3+CD8+, CD16+CD56+ and CD19+ recoveries had no associations with the categorical variables analyzed.
Pre-transplant factors related to lymphocyte recovery.
Abbreviations: ALC, absolute lymphocyte count; BM, bone marrow; MAC, myeloablative; MSD, matched sibling donor; NS, not significant; PBSC, peripheral blood stem cells; RIC, reduced-intensity conditioning; y, years. Statistical analyses were performed using Chi-square or Fisher test.
The 180-day CI of the CMV infection was significantly higher for patients with the ALC and CD3+CD8+ recovered on D+100. Higher infection rates were also observed in females, advanced malignancy, haplo-HSCT and PBSC. In the multivariate analysis, the ALC < 500/µL and CD3+CD8+ < 200/µL effects on the CMV infection remained significant (Table 3).
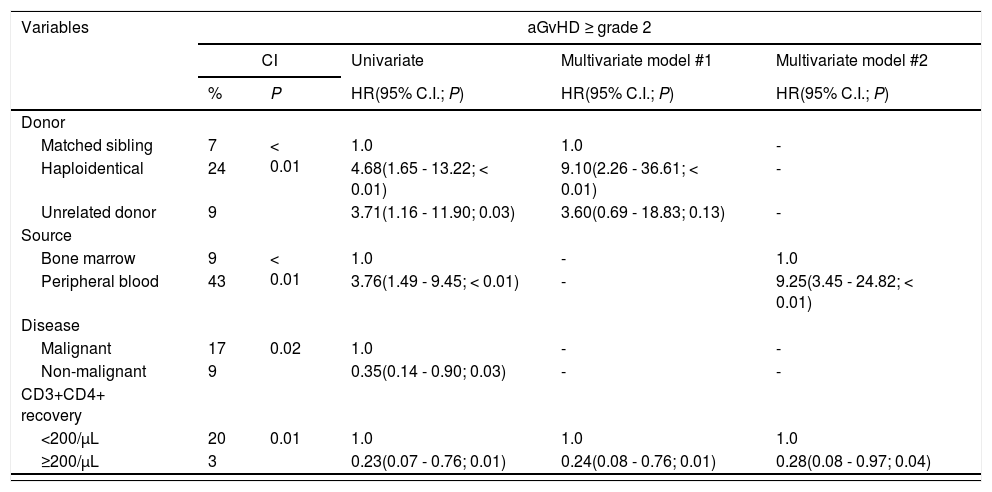
Univariate and multivariate analyses of aGvHD, CMV infection and overall survival.
Statistical analyses were performed using the Gray's test for cumulative incidences; Fine-Gray regression for univariate and multivariate analyses; and Kaplan Meier, log-rank test and Cox regression for survival analysis. Abbreviations: aGvHD, acute graft-versus-host disease; CI, cumulative incidence; CMV, cytomegalovirus; 95% C.I., confidence interval; HR, hazard ratio; P, p-value.
The 1-year CI of the aGvHD ≥ grade 2 was higher in the haplo-HSCT, with PBSC and malignant diseases. Inadequate CD3+CD4+ recovery was the only immune parameter associated with aGvHD. The multivariate analysis revealed that the CD3+CD4+ recovery remained significant when adjusted for the use of the PBSC and haplo-HSCT (Table 3). Relapse of the malignant disease had no association with any immune recovery parameter (data not shown).
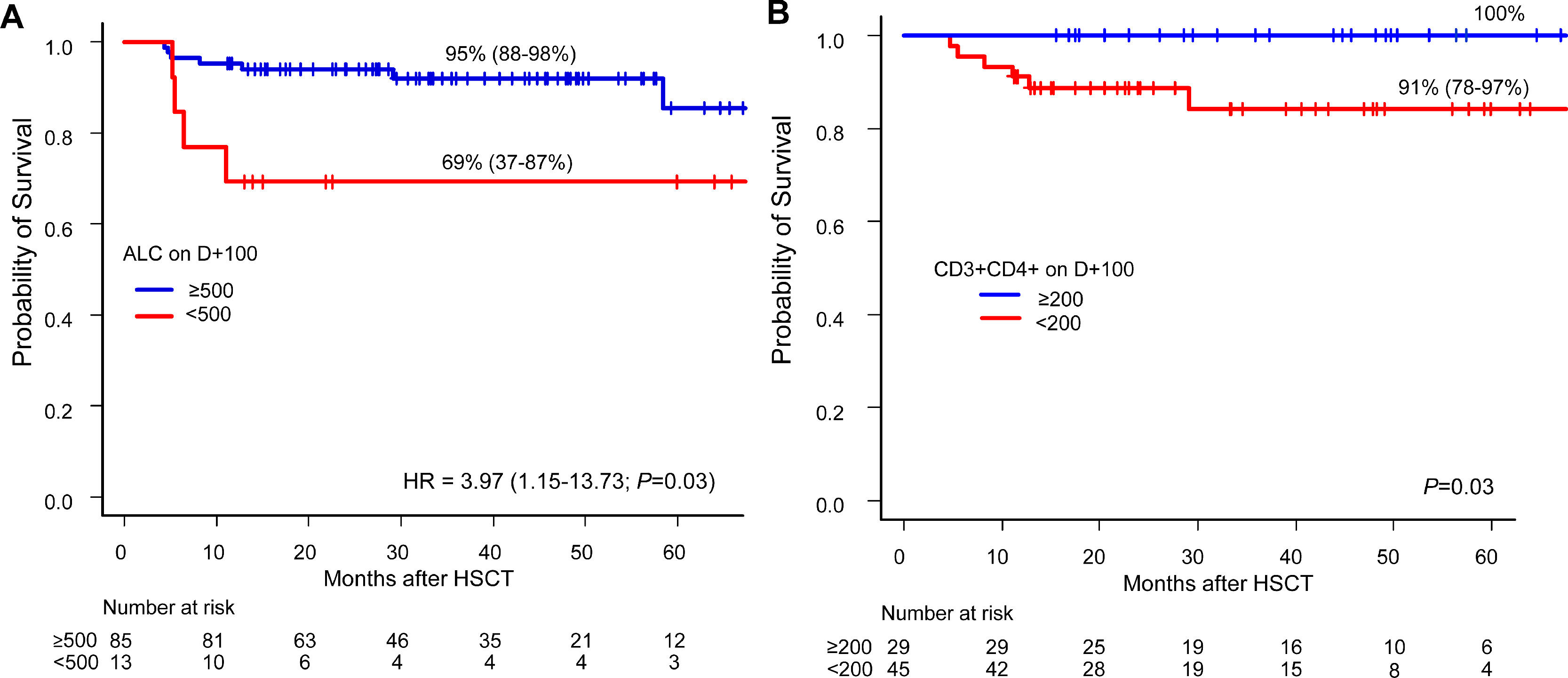
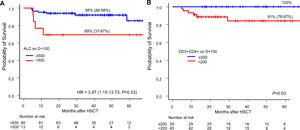
Lymphocyte recovery and survivalOver a median follow-up of 28 months, 22 patients died between days +22 and +1,778. The causes of death were relapse (n = 14), infection (n = 6) and GvHD (n = 2). The factors associated with a higher OS were non-malignant diseases and BM as the stem cell source. The ALC and CD3+CD4+ recovery on D+100 were the lymphocyte parameters associated with higher survival rates (Figure 3). In the multivariate analysis, the protective effect on survival of the ALC recovery on D+100 remained consistent when adjusted for malignant diseases, but it did not reach statistical significance when adjusted for BM grafts (Table 3).
Overall survival according to lymphocyte recovery. A: Overall survival for ALC-recovered patients (blue line) and not recovered (red line) on D+100 (Kaplan-Meier) and its Hazard Ratio by univariate Cox regression. B: Overall survival (Kaplan-Meier) and log-rank of CD3+CD4+-recovered patients (blue line) and not recovered (red line) on D+100. Abbreviations: ALC, absolute lymphocyte count; HR, Hazard ratio.
This study analyzed the lymphocyte recovery after the allo-HSCT in pediatric patients with malignant and non-malignant diseases up to 180 days after the HSCT. Over this 6-month period, the lymphocyte reconstitution was gradual and each subtype showed different reconstitution rates on D+100. Our study reiterates that the lymphocyte count after the HSCT impacts patient survival and is associated with pre-transplant factors, such as age, source of stem cells, donor, serotherapy, conditioning, disease and clinical outcomes, such as the CMV and aGvHD.10-13
In the first three months after the HSCT, natural killer (NK) lymphocytes and the CD3+CD8+ clonal expansion propel the lymphocyte reconstitution.10,14 In our cohort, the ALC recovery occurred possibly due to these subtypes, as the CD3+CD8+ and CD16+CD56+ showed the highest recovery rates on D+100 (68% and 47%, respectively). Kim et al. (2013) also obtained this reconstitution pattern in children and similar median counts for the same period, except for the higher percentage of NK-recovered patients, owing to a lower cut-off value based on the age-matched reference values.15
In this study, patients < 8 years old showed a better recovery for the ALC on D+30 and the CD3+CD4+ on D+100. The higher lymphocyte recovery in younger patients cannot be attributed to the TNC and CD34+ infused cell doses, nor the use of the BM and PBSC, because these factors were statistically similarly distributed in both age groups (data not shown). Previous studies have demonstrated an earlier recovery trend in younger patients through the rapid recovery of the CD3+CD4+, CD19+ and ALC in patients < 5 years old and the CD3+CD8+, CD19+ and CD16+CD56+ in patients < 10 years old.13,15 However, a consensus on this matter is hampered by the distinct lymphocyte recovery criteria according to stem cell sources and age-matched reference values used in published studies.
The higher ALC recovery on D+30 was observed in MAC regimens, compared to the RIC, indicating an attenuated deleterious effect of the MAC on the thymus. In children, this impact might be reduced because the thymus is fully functional until puberty, after which thymopoiesis progressively diminishes.16 Our study showed no association between the conditioning regimen and lymphocyte subtype recovery, possibly due to heterogeneous preparatory regimens. Moreover, the regimen kinetic-dynamics may vary with individual conditions (age and weight), provoking different effects on the IR.17,18
Patients who received BM grafts achieved a higher ALC recovery on D+30. Although the PBSCs mobilized by the granulocyte-colony stimulating factor contain more cells contributing to the lymphocyte count (CD34+, T lymphocytes and NK) and promote the CD3+CD4+ rapid recovery, 93% of the PBSC recipients in this cohort were haplo-HSCT.19,20 Therefore, the donor type may have an impact on the early lymphocyte recovery, regardless of the stem cell source.
We observed that the serotherapy interfered with the ALC recovery on D+180. Inadequate ALC recovery up to 1 year after the HSCT has been described in children.15 A large pediatric cohort showed that high concentrations of ATG after the BM or PBSC transplant reduced the CD3+CD4+ reconstitution. In contrast, in umbilical cord blood transplants, even low ATG concentrations exerted a deleterious effect.21 It is likely that our study revealed no association between lymphocyte subtypes and serotherapy due to the inclusion of only BM and PBSC grafts and the reduced number of patients who used serotherapy.
Viral infections and lymphocyte reconstitution have a complex correlation, as opportunistic infections are related to both the cause and the effect of the delayed IR.22 The literature shows that the impaired CD3+CD8+ early reconstitution contributes to the CMV reactivation.23 But after infection, the clonal expansion of CD3+CD8+ lymphocytes in response to the CMV antigenic stimulus results in an oligoclonal repertoire of memory T cells.24 Thus, the high CD3+CD8+ count observed in patients with the CMV infection, also described by Janecsko et al. (2016), possibly denotes its specific CMV expansion, which could be confirmed in a larger cohort using molecular tests.23
The development of the aGvHD ≥ grade 2 resulted in a lower CD3+CD4+ reconstitution on D+100. Previous reports showed that patients with GvHD had low counts of CD3+CD4+ up to three months after the HSCT, especially naïve CD3+CD4+ cells.25,26 The intense immunodepression is caused by the damage in the thymic function and BM microenvironment, which are essential factors for lymphopoiesis, but can be aggravated by the GvHD treatment with corticosteroids.27 Considering the early development of the aGvHD in this cohort (median day +28), the poor CD3+CD4+ recovery on D+100 may reflect the disease itself and its treatment.
Although relapse of the malignant disease was the major cause of death in our cohort, no association between the relapse and immune recovery parameters was demonstrated due to the limited sample size. However, there is evidence in the literature that the early CD3+CD8+ reconstitution decreases relapse risk, regardless of the diagnosis, conditioning regimen or graft source.28
In our study, the haplo-HSCT had a higher CI of the CMV infection and aGvHD, but the OS was similar to that of other types of transplants. The inadequate ALC recovery on D+30, the lower CD19+ counts and the higher CMV infection risk in the haplo-HSCT may have resulted from the GvHD incidence and immunosuppressive prophylaxis with PT-Cy, CsA and MMF.29 Although major outcomes after the haplo-HSCT are comparable to those of the MSD and matched unrelated HSCT, more studies are needed to better understand the IR in the haplo-HSCT settings, especially for pediatric patients with non-malignant diseases.30
In accordance with a previous study, our data suggest that the ALC ≥ 500/µL on D+100 is a good predictor of the OS for pediatric patients undergoing the allo-HSCT.6 We observed a trend towards the inadequate D+100 CD3+CD4+ recovery in patients dying from severe infections, but the reduced number of patients was insufficient to reveal a significant effect on the OS. However, the CD3+CD4+ is stated to be the most relevant parameter for survival, as it is related to the mortality from infections and aGvHD.25,31,32 A higher survival rate in patients who recovered the ALC and CD3+CD4+ counts may be related to the lower risk of developing severe infections and/or aGvHD. Unfortunately, immunophenotyping requires specialized personnel and costs at least seven times more than the complete blood count (in-house cost), which restrain its regular use in many resource-limited centers. Therefore, simple biomarkers are needed to demonstrate if transplanted patients have achieved a good lymphocyte reconstitution.
Our retrospective study has limitations related to the irregularity in the immune surveillance by flow cytometry after the HSCT, the sample size and the disease heterogeneity. Nevertheless, these real-world data can be extrapolated to other pediatric HSCT centers and form the basis for future collaborative studies. Our study exposes the reality in developing countries, ranging from limited financial resources for laboratory tests, to the failure to comply with the follow-up due to long travel distances to the reference center. Concurrently, as developed countries move towards precision medicine in the IR monitoring, we still endeavor to implement cost-effective strategies to help our patients.
Although only prospective studies compiling multiple parameters may better estimate the immunocompetence timing after the allo-HSCT in resource-limited countries, we need to standardize the IR monitoring with the available tests. In this sense, measuring the ALC monthly after the transplant and lymphocyte subtypes on D+100 and D+180 is feasible to implement in clinical practice to identify patients at risk for an inadequate IR and opportunistic infections. It may guide physicians in managing the antimicrobial prophylaxis, screening pathogens, or introducing adjuvant immunotherapy to boost the immune function.
ConclusionIn conclusion, this study summarized the lymphocyte recovery after the HSCT for malignant and non-malignant diseases in one of the leading pediatric transplant centers in Latin America. The lymphocyte reconstitution was progressive up to D+180, mainly due to the CD3+CD8+. Overall, the younger age, BM grafts, MAC regimens, non-malignant diseases, lack of serotherapy and non-haploidentical HSCT were associated with the adequate lymphocyte recovery. Regardless of the disease type, the ALC ≥ 500/µL on D+100 predicts pediatric survival after the HSCT, preferably concomitant with the lymphocyte subpopulation monitoring.
The authors would like to thank Dr. Euripides Ferreira, the HSCT team and the patients and their families.