Introduction: Systemic Mastocytosis comprises a group of neoplastic diseases characterized by clonal expansion and infiltration of mast cells into several organs. The diagnosis and treatment of this disease may be challenging for non-specialists. Objective: Make suggestions or recommendations in Systemic Mastocytosis based in a panel of Brazilian specialists.
Method and results: An online expert panel with 18 multidisciplinary specialists was convened to propose recommendations on the diagnosis and treatment of Systemic Mastocytosis in Brazil. Recommendations were based on discussions of topics and multiple-choice questions and were graded using the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence Chart.
Conclusion: Twenty-two recommendations or suggestions were proposed based on a literature review and graded according to the findings.
Mastocytosis is a group of neoplastic disorders characterized by expanding and accumulating clonal and neoplastic mast cells (MCs) in the skin and/or various internal organs, such as the bone marrow, spleen, lymph nodes and gastrointestinal tract.1 The updated 2016 World Health Organization broadly classifies mastocytosis into cutaneous and systemic forms. In cutaneous mastocytosis (CM), MCs are restricted to the skin, whereas systemic mastocytosis (SM) refers to systemic involvement of several organs by neoplastic MCs.
The SM is more common in adults than children and is a highly heterogeneous disease, both in clinical presentation and prognosis. Life expectancy for patients with SM can range from normal or near-normal to severely reduced, depending on the aggressiveness of the disease.2
Although published international consensuses of experts on the diagnosis and management of patients with SM exist, there have been no guidelines published on managing this disease in Brazil, a low and middle-income (LMIC) country. This study aims to establish expert panel recommendations regarding the diagnosis and management of SM adapted to the reality of the country.
MethodsEighteen Brazilian experts (including hematologists, immunologists and dermatologists) met online on May 15, 2021, to discuss relevant clinical questions regarding the diagnosis, treatment, and follow-up of patients with SM. Thirty multiple-choice questions, previously defined by 5 of these specialists (area coordinators), were submitted for discussion and voting.
A recommendation was established if at least 75% of the panel agreed with an answer. A suggestion was presented if an agreement of less than 75%, but greater than 49%, was achieved. No recommendations or suggestions were made if there was less than 50% agreement.
The Oxford 2011 Levels of Evidence3 document was used to grade the expert panel recommendations and suggestions.
Results and discussionThe questions used to elaborate these recommendations and the numbers of votes can be found in Supplementary Table 1.
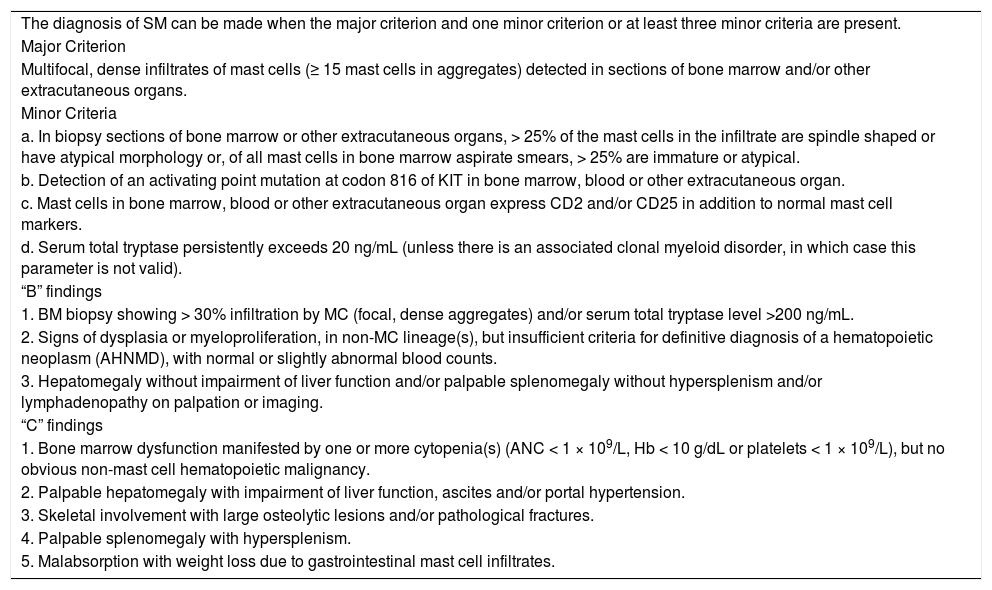
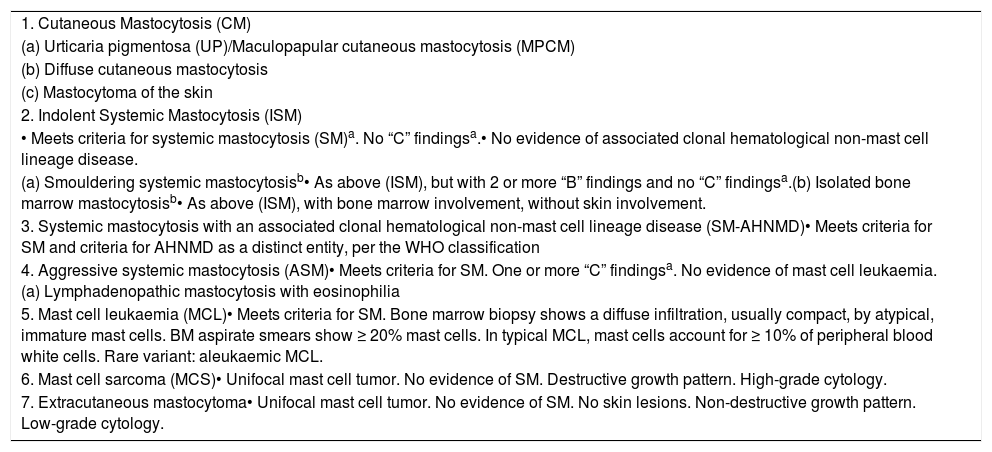
Diagnosis and classificationMastocytosis is a clonal proliferation of abnormal MCs that accumulate in one or more organs.4 The clinical presentation of mastocytosis is heterogeneous, ranging from skin-limited disease (CM) to a more aggressive variant with extra-cutaneous involvement (SM). Criteria for diagnosis were developed by the World Health Organization (WHO) in 2001, with the last update in 2016.5Table 1 shows the current WHO criteria for SM diagnosis and Table 2, the current mastocytosis classification. Figure 1 illustrates several clinical and laboratory features of this disease.
World Health Organization (WHO) diagnostic criteria for systemic mastocytosis.
Adapted from Ref. 4.
World Health Organization (WHO) classification of mastocytosis.
| 1. Cutaneous Mastocytosis (CM) |
| (a) Urticaria pigmentosa (UP)/Maculopapular cutaneous mastocytosis (MPCM) |
| (b) Diffuse cutaneous mastocytosis |
| (c) Mastocytoma of the skin |
| 2. Indolent Systemic Mastocytosis (ISM) |
| • Meets criteria for systemic mastocytosis (SM)a. No “C” findingsa.• No evidence of associated clonal hematological non-mast cell lineage disease. |
| (a) Smouldering systemic mastocytosisb• As above (ISM), but with 2 or more “B” findings and no “C” findingsa.(b) Isolated bone marrow mastocytosisb• As above (ISM), with bone marrow involvement, without skin involvement. |
| 3. Systemic mastocytosis with an associated clonal hematological non-mast cell lineage disease (SM-AHNMD)• Meets criteria for SM and criteria for AHNMD as a distinct entity, per the WHO classification |
| 4. Aggressive systemic mastocytosis (ASM)• Meets criteria for SM. One or more “C” findingsa. No evidence of mast cell leukaemia.(a) Lymphadenopathic mastocytosis with eosinophilia |
| 5. Mast cell leukaemia (MCL)• Meets criteria for SM. Bone marrow biopsy shows a diffuse infiltration, usually compact, by atypical, immature mast cells. BM aspirate smears show ≥ 20% mast cells. In typical MCL, mast cells account for ≥ 10% of peripheral blood white cells. Rare variant: aleukaemic MCL. |
| 6. Mast cell sarcoma (MCS)• Unifocal mast cell tumor. No evidence of SM. Destructive growth pattern. High-grade cytology. |
| 7. Extracutaneous mastocytoma• Unifocal mast cell tumor. No evidence of SM. No skin lesions. Non-destructive growth pattern. Low-grade cytology. |
Adapted from Ref. 4.
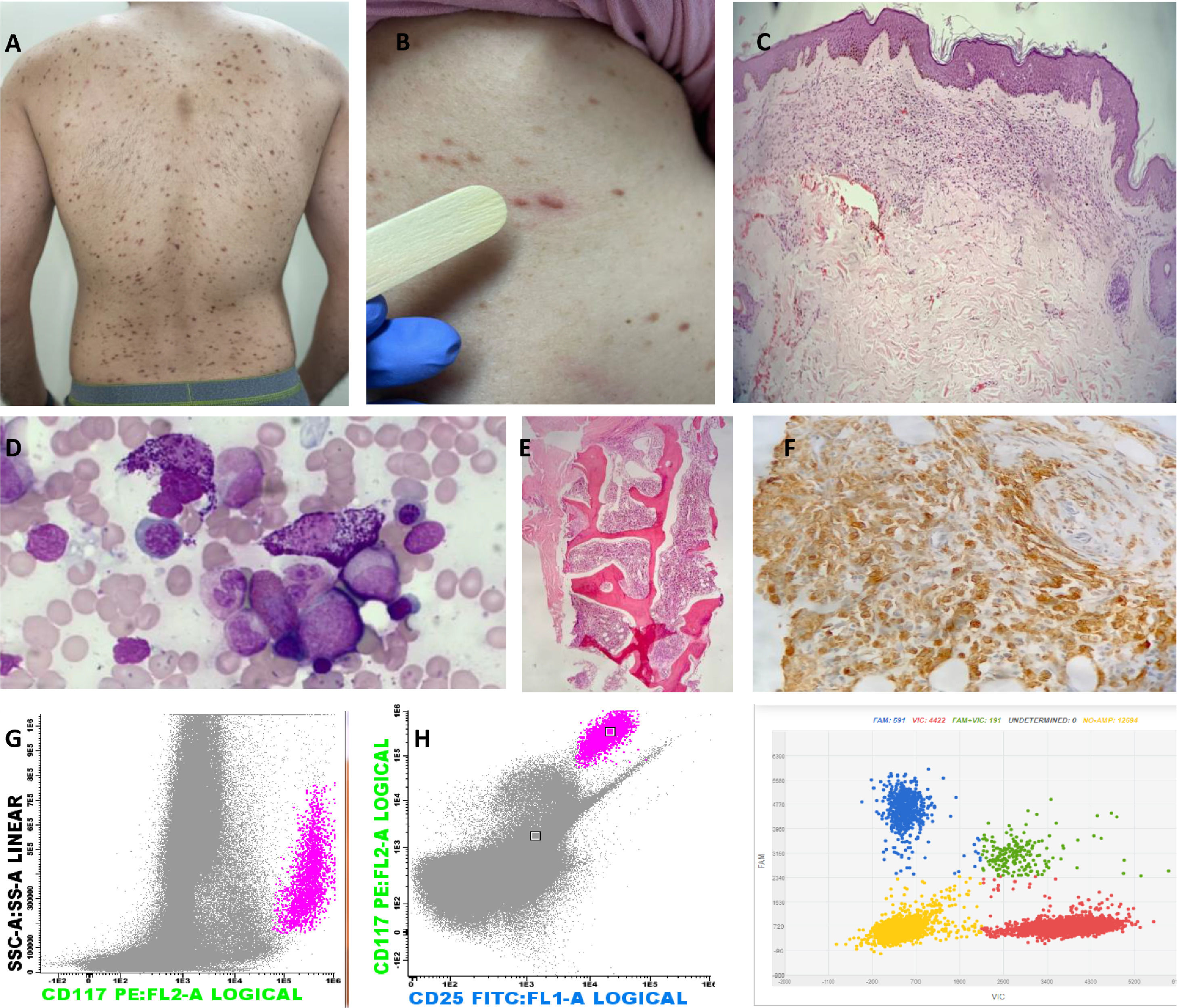
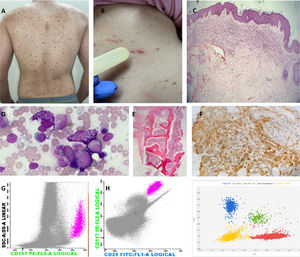
Images representing major and minor WHO criteria for the diagnosis of SM and cutaneous involvement. (A) Red–brownish maculopapular cutaneous lesions, monomorphic type (formerly known as urticaria pigmentosa) in patients with ISM. (B) Positive Darier's sign – A wheal-and-flare reaction develops upon stroking of a CM lesion with a tongue spatula. (C) The dermal cellular infiltrate consists predominantly of mast cells associated with vascular congestion and mild fibroplasia (H&E, 40x). (D) Bone marrow (BM) aspirate – anomalous hypogranular and spindle-shaped mast cells (Leishman, 200x). (E) BM biopsy – global hypercellularity (95%), extensive infiltration by mast cells. Bone BM trabeculae without significant histological changes (H&E, 200x). (F) Immunohistochemistry of BM shows a large mast cell burden (CD117 immunostaining counterstained with Harris hematoxylin, 400x). (G) Flow cytometry of BM cells shows anomalous CD25+ mast cells (pink dots); mast cells are identified through CD117 positivity and the high internal complexity of the cells, anomalous phenotype CD117+/CD25+. (H) Digital PCR (peripheral blood analysis) for the D816V mutation. Red represents the wild-type codon and blue represents the D816V mutation. Two copies are represented together in green (wild-type and mutated); the yellow color represents the absence of the studied gene.
A small study of 24 patients6 found that, although all WHO criteria have high specificity (100%), the sensitivity of individual criteria varied from 69% for bone marrow biopsy histopathologic findings to 92% for the presence of an aberrant phenotype in the bone marrow aspirate. These findings led to low negative predictive values in the studied population, ranging from 38% to 75% for the same criteria mentioned above.
The sensitivity of the KIT mutation testing for the SM diagnosis may be enhanced by enriching the sample for abnormal MCs by using laser capture microdissection,7 magnetic bead-based, FACS-based cell sorting and other techniques with higher sensitivity.8
Suggestion: New or modified diagnostic criteria with higher sensitivity should be included in future revisions of the WHO criteria to diagnose Systemic Mastocytosis (GRADE 3).
It is estimated that most adult patients with mastocytosis have SM,9 but that isolated cutaneous disease is more prevalent in pediatric patients.10 Skin manifestations are observed in approximately 80% of adult patients with SM,11 but a specific population may present with a subvariant form of indolent SM (ISM) solely with bone marrow involvement.9 The SM should always be suspected and investigated in adult patients with cutaneous disease.12
A bone marrow biopsy is rarely performed for children,9 but a recent review suggests that the systemic involvement should be investigated in selected cases.13
Cutaneous Mastocytosis (CM) is defined by typical skin lesions of mastocytosis associated with Darier's sign (major criterion) and one or two of the following criteria1415:
- 1.
Increased numbers of mast cells in biopsy sections of lesional skin (approximately 40 mast cells/mm2), and;
- 2.
(Activating) KIT mutation in skin lesion. However, it should be noted that at present, only a few laboratories are able to sequence KIT from skin tissue biopsy.
Dariers sign is elicited by stroking a mastocytosis skin lesion approximately 5 times by using moderate pressure with a tongue spatula. Within a few minutes, a wheal-and-flare reaction of the lesion (not or hardly seen in the surrounding skin) will develop.14
The CM is classified based on macroscopic features and the pattern of the distribution of skin lesions. In contrast to Maculopapular Cutaneous Mastocytosis (MPCM) and diffuse CM, mastocytomas are almost never observed in adults.16
Patients with SM often also present with cutaneous involvement, which is characterized by erythematous-brown fixed maculopapular lesions. Adults with ISM usually manifest monomorphic maculopapular lesions, whereas children with CM develop polymorphic maculopapular lesions. Maculopapular skin lesions found in patients with ISM and those with CM also occur in patients with advanced SM. It is estimated that approximately 80% of patients with ISM exhibit maculopapular skin lesions, compared to only approximately 50% of patients with advanced SM.16
Recommendation: Cutaneous Mastocytosis should be diagnosed only after the dermatological clinical evaluation, anatomopathological examination and exclusion of Systemic Mastocytosis criteria (GRADE 1).
Tryptase is a serine protease predominantly produced by tissue mast cells and is the most specific marker of MC activation and burden.16 Elevated serum tryptase can be found in several clinical conditions and diseases.16,17
In SM, a persistently elevated serum tryptase level (> 20 ng/ml) is a minor diagnostic criterion in the WHO diagnostic framework5; levels vary widely, but serum tryptase is elevated in the vast majority of SM patients across all WHO subgroups. A significantly greater proportion of Aggressive Systemic Mastocytosis (ASM) and Systemic Mastocytosis with an Associated Clonal Hematological Non-Mast Cell Lineage Neoplasm (SM-AHN) patients exhibit a markedly more elevated serum tryptase level (> 200 ng/ml) than those with ISM.4 Patients with CM, except for cases with extensive skin involvement, typically exhibit normal levels of total tryptase and serum tryptase has been shown to be a sensitive marker for the SM diagnosis.18–20
Serum basal levels of tryptase also correlate with disease progression and response to treatment21 and, therefore, should be tested periodically, particularly in advanced cases.
Recommendation: Tryptase level measurements are necessary for the Systemic Mastocytosis diagnosis and follow-up (GRADE 2) at least once a year (GRADE 5).
In patients with SM, the bone marrow is the organ most targeted by MCs. The evaluation of bone marrow, including the bone marrow aspirate, immunophenotyping by flow cytometry and histopathological evaluation of the bone marrow biopsy, can detect infiltration by neoplastic, abnormal MCs and is one of the cornerstones for the diagnosis of SM by the WHO SM criteria.4,5 Indeed, according to the 2016 WHO Classification, detection of multifocal dense infiltrates of MCs (≥ 15 mast cells in aggregates) in the bone marrow is a major criterion for the SM diagnosis. The presence of atypical morphology in more than 25% of the MCs, including spindle-shaped MCs, is considered a minor criterion.5 Thus, bone marrow evaluation by a unilateral bone marrow biopsy with a histopathological review, preferably by a pathologist with expertise in recognizing MC disorders, is mandatory.4,5,22
Moreover, the immunophenotyping evaluation of the MCs can help detect small infiltrates that are not readily apparent in the morphological evaluation of the marrow biopsy.23,24 The immunohistochemical staining for CD117 and tryptase can readily detect the presence of small MC aggregates, but cannot distinguish between normal and neoplastic MCs.25 The expression of the CD25 and/or CD2 can help in the differentiation of normal (negative for these markers) and neoplastic (positive for these markers) MCs25 and the expression of CD25 and CD2 is also considered a minor criterion by the 2016 WHO Classification.2 Of the two markers, the CD25 appears to be the most sensitive, being found in most cases of both advanced and indolent SM.26 The CD25 and CD2 can be evaluated by immunohistochemistry in the marrow biopsy and/or by flow cytometry.
Most patients with systemic mastocytosis harbor mutations in the KIT gene, which encodes the stem cell factor receptor CD117, a class III receptor tyrosine kinase expressed by MCs, hematopoietic progenitor cells, germ cells, melanocytes and interstitial cells of Cajal in the gastrointestinal tract.27 The gain-of-function D816V mutation in the KIT gene is detected in most (> 95%) adult patients with SM.28 Other KIT mutations are found in pediatric patients with CM29 and a minority of adult SM patients.4 Some of these other KIT mutations and the rare case of SM with wild-type KIT may involve disease that is responsive to the tyrosine kinase inhibitor imatinib.30,31
The D816V KIT mutation allele burden was also found to correlate with disease activity, disease subtype and survival.28,3233 This mutation confers the receptor with a conformational modification, rendering cells resistant to imatinib.34 Some patients with increased MCs and eosinophilia may carry the FIP1L1-PDGFRA fusion gene associated with imatinib sensitivity.35 However, it should be mentioned that, according to the 2016 WHO Classification, such a disease would be classified as the Myeloid/Lymphoid Neoplasm with PDGFRA rearrangement, and not SM.5
Analysis of KIT mutations in bone marrow cells is a standard diagnostic procedure for SM. In most patients with SM, particularly those with ISM, the burden of neoplastic MCs in the bone marrow can be very low and this may represent a technical challenge in detecting the mutation, if only conventional detection methods are employed (e.g., Sanger sequencing).36 The sensitivity of the KIT mutation testing for the SM diagnosis may be enhanced by enriching the sample for neoplastic MCs by laser capture microdissection, magnetic bead-based, FACS-based cell sorting, or using digital/allele-specific PCR techniques with high sensitivity.8–37
Recommendation: The D816V KIT mutations should be investigated in all patients with Systemic Mastocytosis. The FIP1L1-PDGFRA fusion gene needs to be investigated in patients with increased BM mast cells and eosinophilia (GRADE 2).
Imaging studies can determine the degree of mast cell infiltration in specific organs. Magnetic resonance imaging (MRI) is more sensitive than conventional X-rays in detecting bone marrow involvement and a whole-body MRI exam may also contribute to determining the presence of B/C-finding factors, such as the presence of hepatosplenomegaly and ascites.38
The most common bone abnormality in patients with SM is diffuse demineralization, which can be revealed by the bone mineral density (BMD) of the lumbar spine and femur with the dual-energy X-ray absorptiometry (DEXA) technique.38 The Tc99 bone scintigraphy can detect diffuse bone involvement and a greater number of focal lesions, with a higher sensitivity than plain radiography.39
Infiltration of the intestinal tract by MCs can be confirmed by biopsy during colonoscopy, but bowel involvement rarely correlates with symptoms.40 Abdominal ultrasonography is the most common initial study in patients with SM to assess abdominal involvement.38 Computed tomography (CT) scans are also used to detect abdominal signs of MC infiltration in the liver, spleen and other abdominal organs.3841 Although the findings in patients with SM are not specific, they may be used to direct further studies for diagnostic confirmation and to estimate the extent of systemic involvement.42
Positron emission tomography/computed tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (FDG-PET/CT scan) has been shown in a multicenter study43 and in a case series of five SM patients44 to have little value in diagnosing or staging most forms of the disease. Higher uptake was detected in patients with SM-AHN, mast cell sarcoma (MCS)43 and in a single case report of an MCL patient with extramedullary involvement.45
Recommendation: Doppler abdominal ultrasound should be performed for all patients with Systemic Mastocytosis to evaluate hepatomegaly, portal hypertension and ascites (GRADE 3).
Recommendation: CT scans and MRI should be used to evaluate Systemic Mastocytosis “C” findings. The FDG-PET/CT scans should not be part of the initial assessment of the most common forms of the disease (GRADE 3).
PrognosisThe 2016 WHO classification of Systemic Mastocytosis is the most practical first step in determining prognosis for each case.4 A study with 342 patients performed at the Mayo Clinic46 validated the prognostic relevance of the WHO classification system; the multivariable analysis showed a significant and independent association of inferior survival with WHO subtype (p < 0.001), advanced age (p < 0.001), weight loss (p < 0.01), anemia (p < 0.007), thrombocytopaenia (p < 0.001), hypoalbuminemia (p < 0.001) and excess BM blasts (> 5%; p < 0.004). In a more recent analysis of 580 patients, the Mayo Alliance Prognostic System (MAPS) developed a hybrid clinical-molecular model that included age > 60 years, WHO-defined advanced SM, thrombocytopenia < 150 × 109/L, increased serum ALP and anemia (defined as a hemoglobin level below the sex-adjusted normal reference range) or adverse mutations (ASXL1, RUNX1 and NRAS). The survival correlated directly and proportionally with the number of risk factors.47
Recommendation: The clinical or hybrid Mayo criteria should be used to determine prognosis (GRADE 4).
Treatment and follow-upThe treatment of SM should be individualized and varies from a watch-and-wait period to the symptom management, supportive measures and cytoreductive therapy for MC debulking in the setting of aggressive, advanced or treatment-refractory disease.4 Allogeneic stem cell transplantation is another option for patients with SM-AHN or ASM.48,49
The symptomatic treatment aims to manage symptoms efficiently and minimize their recurrence. The avoidance of triggers and use of prophylactic medication, when risk avoidance is difficult or impossible, anre necessary first measures for symptomatic control.49 Mastocytosis symptoms can be divided into skin symptoms, mast cell mediator “release” symptoms and symptoms caused by non-cutaneous organ infiltration.50
The treatment of mild clinical manifestations is mainly symptomatic and consists of the following: H1-histamine receptor blockers for general symptoms; H2-histamine receptor blockers, mainly for gastrointestinal symptoms, and; leukotriene receptor blockers and mast cell stabilizers.49 Glucocorticoids may be useful in treating SM for acute reactions, though long-term adverse effects may limit their use. Cutaneous manifestations may also respond to topical corticosteroids and calcineurin inhibitors.51 See Table 3 for the treatment of cutaneous symptoms in SM.
Principles of treatment of cutaneous involvement in systemic mastocytosis.
| General measures - ALL PATIENTS |
| 1. Avoidance of Triggers• Avoid medication that directly activate mast cells, allergens, Hymenoptera stings and other triggers§• Physical stimulusAvoid sudden changes and extreme temperatures in bath/shower, swimming pool, air conditioning and environmentAvoid rubbing |
| 2. Skin care• Avoid dryness of skin• Use skin moisturizer |
| 3. Photoprotection |
| Regular Medications for Symptomatic Patients1. Maculopapular skin lesions, itching and flushing• H1 antihistamines (first and second generation)• H2 antihistamines |
| 2. Continuous moderate symptoms• Scheduled non-sedating H1 antihistamines, add sedating H1 antihistamines on demand• Scheduled or on demand H2 antihistamines (e.g., famotidine)• Mast cell membrane stabilizers& |
| 3. Severe symptoms• All of the above• Add anti-leukotrienes (e.g., montelukast) |
| Alternative treatments1. Topical• Corticosteroid cream*• Topical calcineurin inhibitors+• Sterile gauze with zinc sulphate# |
| 2. Systemic treatment• Corticosteroids* |
| 3. Physical treatment• Phototherapy – second-line• Lasers (Dye-lasers/Nd-Yag) |
| 4. Surgery• Solitary Mastocytoma could be considered for excision as a third line treatment. |
Ketotifen ± oral or cream l disodium cromolyn (the commercial form is only available in manipulation pharmacies at a high cost in Brazil).
In general, may reduce skin symptoms and lead to cosmetic improvement. Nevertheless, these medicines should be prescribed only for short-term therapy due to numerous skin side effects, including cutaneous atrophy, telangiectasia, hyperpigmentation, hypopigmentation and systemic side effects, such as osteopaenia, or adrenocortical suppression in repeated or extensive application.
Brazzelli et al52. published a research report on 20 patients with CM and ISM treated with the PUVA therapy (UVA plus psoralen therapy) and NB-UVB (narrowband UVB), with good results. In general, however, it is a method of limited efficacy and only partial and temporary improvement of skin signs and symptoms can be achieved. The carcinogenic effect of phototherapy and photochemotherapy should also be considered, particularly with long-term or recurrent ultraviolet phototherapy, which is needed to achieve a skin response in CM.
The melanoma incidence seems to be elevated in patients with mastocytosis53 and careful monitoring of premalignant skin lesions should be carried out periodically.54
Recommendation: The treatment of cutaneous disease should focus on symptomatic relief and trigger avoidance (GRADE 2).
Recommendation: Antihistamines should be used for the symptomatic treatment (GRADE 2).
Suggestion: Antihistamines may be used for prophylactic treatment, especially in highly symptomatic patients (GRADE 2).
Recommendation: The follow-up in patients with indolent or smouldering Systemic Mastocytosis should include symptomatic management and bone disease measurement (GRADE 3).
Recommendation: A dermatological examination should be performed at least once a year. Emollients, insect repellents, H1 ± H2 antihistamines, sunscreens, ketotifen and cromoglycate* are the basis of treatment (GRADE 4) *(GRADE 2).
The anaphylaxis is a significant complication in mastocytosis patients. Although the risk of anaphylaxis is less than 10% in children with CM, it is estimated to be 50% in adults with ISM.55 Anaphylactic reactions may be elicited by drugs, hymenopteran (bee, wasp and ant) venom, physical factors, such as heat and friction and infection, among other inducers.56 The risk of anaphylaxis should be evaluated prior to a medical procedure to determine the need for premedication, as proposed by Hermans et al.57 Risk factors for complications depend on the procedure (use of general anesthesia and major surgery) and patient clinical features (history of anaphylaxis, use of medication, severe skin infiltration, etc.).56,57
The anaphylaxis may be induced by allergic (IgE-mediated) and non-allergic mechanisms (direct mast cell activation).58,59 Patients with mastocytosis have a higher prevalence of hymenopteran venom allergy, but not of drug or food allergy, in comparison to the general population.60 The Hymenoptera (bees, wasps and ants) acts through both allergic IgE-mediated mechanisms and direct mast cell activation.61 There is a specific ISM patient phenotype, with no cutaneous involvement and anaphylactic shock, induced by the hymenopteran venom.62 Patients with SM and allergies to Hymenoptera should undergo lifelong immunotherapy.
Drugs that directly activate mast cells may induce non-allergic anaphylactic reactions in mastocytosis patients63,64 and these drugs should be used with the utmost caution (Table 4). A Spanish study of 501 mastocytosis patients who underwent 726 anesthetic procedures showed that MC mediator-related symptoms and anaphylaxis were present in 2% and 0.4% of adults and 4% and 2% of children, respectively.65 Neuromuscular blocking agents and opioids are the main drugs involved in reactions in these situations. An ENDA/EAACI systematic review did not find clear evidence of higher risks with general or local anesthetics, beta-lactam antibiotics and radio-contrast media.66
Drugs that directly activate mast cells and should be prescribed with caution, only if inevitable.
| Opioids |
| Neuromuscular blocking agents |
| Quinolones and Vancomycin |
| Iodinated contrast media |
| Non-steroid anti-inflammatory drugs (NSAIDs)* |
| Hermans et al. showed that the risk is not very high.62 Guidance must be personalized and patients taking NSAIDs without having adverse reactions can continue to use them. |
In children with mastocytosis, the percentage of anaphylaxis has been reported to be between 6% and 9%, but adults have a much higher incidence (22% to 49%).55 In a study of 120 patients, including children and adults,60 major perceived trigger factors for adults were hymenopteran stings (19%), foods (16%) and medications (9%); however, in 26% of the reactions, only a combination of different triggers preceded the anaphylaxis. Trigger factors remained unidentified in 67% of the reactions in children, compared to 13% in adults. Patients with anaphylaxis had higher basal tryptase values (60.2 ± 55 ng/ml, p < 0.0001) than those without anaphylaxis (21.2 ± 33 ng/ml). In another study67 of adult SM patients, 36 of 84 patients were identified as having had at least one episode of an anaphylactic reaction (43%); 22 patients had single episodes, with the remaining 14 patients having 55 episodes. Reactions without known triggers, that is, idiopathic reactions, were also common in this cohort, totaling 39% (14/36). In contrast, only three patients had a history of anaphylaxis after ingestion of foods (one case) or drugs (two cases).
Recommendation: The use of prophylaxis before procedures should be individualized, depending on the procedure and the form of the disease (GRADE 1). The premedication before the skin biopsy is usually not necessary. However, the use of premedication to decrease the risk of anaphylaxis before invasive procedures, such as bone marrow biopsy and endoscopy, is recommended.
The self-injectable epinephrine (two doses) should be carried by all patients with SM all the time, even if previous anaphylaxis has not occurred. Both the patient and family members/caregivers should be trained in administering epinephrine.60,68–71
Recommendation: The intramuscular epinephrine should be used as an emergency treatment for anaphylactic reactions (GRADE 5).
Recommendation: Beta-lactam antibiotics and local anesthetics can be safely prescribed for Systemic Mastocytosis patients (GRADE 1).
The authors suggest that patients with a known tolerance to NSAIDs can continue the treatment, whereas a diagnostic workup should be performed for those with a prior reaction to NSAIDs.66
Recommendation: The NSAID indications should be individualized for each patient (GRADE 1).
In a survey by the Mastocytosis Society, over half of the mastocytosis patients reported allergic symptoms after ingestion of certain foods and beverages.72 On the other hand, Jarkvist et al.73 analyzed complete allergic workups in 187 SM patients and found that the prevalence of food hypersensitivity reactions or food allergies was the same as that in the general population (17.2% and 3.4%, respectively).
Recommendation: Systemic Mastocytosis patients have the same frequency of food allergies as the general population and there is no need for a prior food restriction due to potential allergies (GRADE 2).
Mast cell mediators, mainly interleukin-6, affect the bone metabolism74 and the presence of osteoporosis in SM patients varies from 8% to 41%.75 Fragility fractures due to osteoporosis are also common, particularly in men76 and patients without skin involvement77 with ISM. The bone density may be increased with denosumab78 or zoledronic acid.79 Vitamin D and calcium were included in a bisphosphonate regimen in another study of 23 patients with ISM and osteoporosis, showing increased bone mineral density in all evaluated patients (9/9).80 Antiresorptive drugs reduce the incidence of fragility fractures in these patients, though they may still occur in patients with a previous fracture occurrence.81
Recommendation: Bisphosphonates and vitamin D, plus calcium treatment, are recommended for SM patients with osteopenia/osteoporosis (T score ≤ 2) (GRADE 4). Patients with osteoporosis should be referred to a specialist.
Recommendation: The cytoreductive therapy is indicated in patients with pathological fractures (GRADE 5).
The ASM has a worse prognosis than ISM, presenting with “C” findings (Table 1), organ dysfunctions and, less frequently, cutaneous disease.82 The cytoreductive treatment is indicated for these patients, in whom the need to control the myeloproliferation and reduce damage to target organs outweighs the potential side effects of therapies.83 The choice of the most appropriate therapy should be determined, based on the clinical picture and molecular data (particularly the KIT mutational status and additional high-risk mutations).83
The imatinib, a competitive inhibitor of several tyrosine kinases, including the KIT, has been tested in SM patients, with disappointing results.84,85 The imatinib inhibits the growth of cells with the wild-type KIT or V560G KIT, but not cells bearing the D816V KIT mutation. The resistance to imatinib in patients with the KIT D816V mutation may result from a conformational change in the activation loop located at the entrance to the KIT enzymatic pocket. The conformational change interferes with imatinib binding to the receptor, rendering the cells resistant to its effect.86 In selected patients with SM and KIT non-D816V mutations, the use of imatinib may lead to good results.30,31,87
Recommendation: The imatinib is a treatment option for patients with recently diagnosed aggressive SM and a negative test for the D816V KIT mutation or in the presence of other mutations known to be sensitive to imatinib (or in situations in which genetic tests are not available) (GRADE 4).
The interferon-α (IFN-α) has been tested in 76 SM patients in 6 different studies from 2002 to 2009 (reviewed by Weis Bjerrum et al.88). Complete responses were seen in 7% and 46% responded partially. Adverse reactions are a significant hurdle for long-term use of the IFN-α in the treatment of patients with SM and the dose escalation may be difficult to achieve.88
Although MC mediators are involved in several aspects of pregnancy, little is known about the effects of SM in pregnancy and vice versa.88 The treatment should be directed towards relieving SM symptoms, while weighing the risks of medications to the fetus. The use of the IFN-α for the treatment of other conditions during pregnancy was not associated with maternal or fetal complications or malformations and its use may be considered for pregnant women who need cytoreductive therapy.89
Recommendation: The interferon-alpha should be considered for pregnant patients with advanced systemic mastocytosis (GRADE 4).
The cladribine has been evaluated in two retrospective studies,90,91 showing partial responses in 72% and 50% of the patients. One patient in the study of Lim et al.91 experienced a complete response. Patients with less aggressive disease had a better response rate in the Barete et al. study,90 with ISM (89%), SSM (100%) and CM (100%), compared to ASM (43%) and SM-AHNMD (59%) (p < 0.001). The main adverse events in both studies were myelosuppression and infection. The cladribine may also be helpful in patients with ISM or SSM with refractory symptoms due to mast cell mediator release or bone disease not responsive to anti-mediator drug therapy or bisphosphonates.92
The advanced SM (comprising patients with ASM, SM-AHN and MCL) treatment with midostaurin has been shown to deliver good results in an open-label study with 116 patients,93 with a 60% overall response rate, but no difference in the response according to the disease subtype. The dose reduction, owing to toxic effects, occurred in 56% of the patients and the re-escalation to the starting dose was feasible in 32% of those patients. Another phase II study94 evaluated 26 ASM patients treated with midostaurin; after 10 years of follow-up, in addition to the 69% response rate in the first report,95 2 others achieved complete responses with continuing treatment. The responses were durable and associated with symptom improvement and significant decreases in bone marrow MC burden and serum tryptase levels. In comparison to historical controls, it has been suggested that midostaurin prolongs overall and progression-free survival.4 Low-grade gastrointestinal symptoms were the most common adverse reactions and were manageable with symptomatic treatment. Overall, treatment with midostaurin reduces disease burden, as demonstrated by a decrease in serum tryptase and MC numbers and reduced spleen size in patients with splenomegaly.95 In a phase 2 trial with 116 patients,96 midostaurin also improved symptoms related to SM, with a decrease in symptom prevalence. The median progression-free survival was 14.1 months and the median overall survival, 33.1 months.
To date, despite no randomized clinical trials comparing midostaurin to other cytoreductive agents, a non-randomized comparison of midostaurin to a historical cohort suggested improvement in survival outcomes with this agent.97
At the time of the writing of this article, avapritinib, a new tyrosine-kinase inhibitor approved by the FDA in the USA for SM therapy, was not currently available in Brazil.98Table 5 shows the cytoreductive agents used for Systemic Mastocytosis.
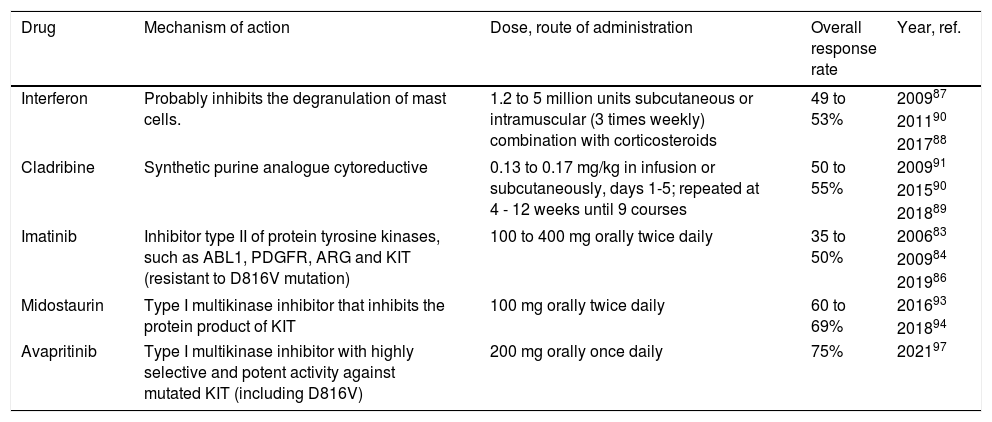
Cytoreductive agents in systemic mastocytosis.
| Drug | Mechanism of action | Dose, route of administration | Overall response rate | Year, ref. |
|---|---|---|---|---|
| Interferon | Probably inhibits the degranulation of mast cells. | 1.2 to 5 million units subcutaneous or intramuscular (3 times weekly) combination with corticosteroids | 49 to 53% | 200987 |
| 201190 | ||||
| 201788 | ||||
| Cladribine | Synthetic purine analogue cytoreductive | 0.13 to 0.17 mg/kg in infusion or subcutaneously, days 1-5; repeated at 4 - 12 weeks until 9 courses | 50 to 55% | 200991 |
| 201590 | ||||
| 201889 | ||||
| Imatinib | Inhibitor type II of protein tyrosine kinases, such as ABL1, PDGFR, ARG and KIT (resistant to D816V mutation) | 100 to 400 mg orally twice daily | 35 to 50% | 200683 |
| 200984 | ||||
| 201986 | ||||
| Midostaurin | Type I multikinase inhibitor that inhibits the protein product of KIT | 100 mg orally twice daily | 60 to 69% | 201693 |
| 201894 | ||||
| Avapritinib | Type I multikinase inhibitor with highly selective and potent activity against mutated KIT (including D816V) | 200 mg orally once daily | 75% | 202197 |
Recommendation: The cytoreductive therapy is indicated for patients with advanced systemic mastocytosis and for patients with indolent or smouldering Systemic Mastocytosis, for whom symptom therapy fails. There are no randomized trials of different cytoreductive agents that are commercially available in Brazil. The cladribine, IFN-α and midostaurin are appropriate first-line therapeutic choices for cytoreductive therapy (GRADE 5).
The allogeneic stem cell transplantation should be considered as the treatment for patients with advanced SM. The decision to proceed to the allogeneic stem cell transplantation should be individualized for each case, considering the patient age and comorbidities, disease prognosis (expected survival inferior to 5 years), donor availability and the prognosis of the associated hematological neoplasm in patients with SM-AHN.48,92
Recommendation: The allogeneic stem cell transplantation should be considered in eligible patients with advanced SM with an estimated survival of fewer than 5 years and/or patients with SM-AHN with hematological neoplasms who have an indication for allogeneic stem cell transplantation (GRADE 4).
In patients with SM-AHN, clinical, histological and molecular data should be integrated to assess which component requires more immediate treatment.4 Briefly, in patients with associated aggressive neoplasms, such as advanced myelodysplastic syndrome or acute myeloid leukaemia, the treatment should focus on the associated neoplasm. If symptoms and complications are deemed to be related to the SM, the treatment should focus on the SM component.
Recommendation: For patients with SM-ANH, the treatment focus should be the most symptomatic disease, considering the allogeneic stem cell transplant, based on the disease risk and comorbidities (GRADE 5).
Response criteria for the SM treatment have recently been reviewed.99 The most recent models (the IWG-MRT-ECNM and modified IWG-MRT-ECNM) were developed to build upon and overcome the limitations of the prior response criteria.100 The IWG criteria include comprehensive definitions of organ damage eligible for the response evaluation, using the CTCAE grading; the organ dysfunction is required to be grade 2 and specific criteria for clinical improvement are defined.
Suggestion: The criteria for response should be individualized, taking into consideration the "C" findings, serum tryptase, D816V allele burden, bone marrow mastocyte infiltration and quality of life (GRADE 4).
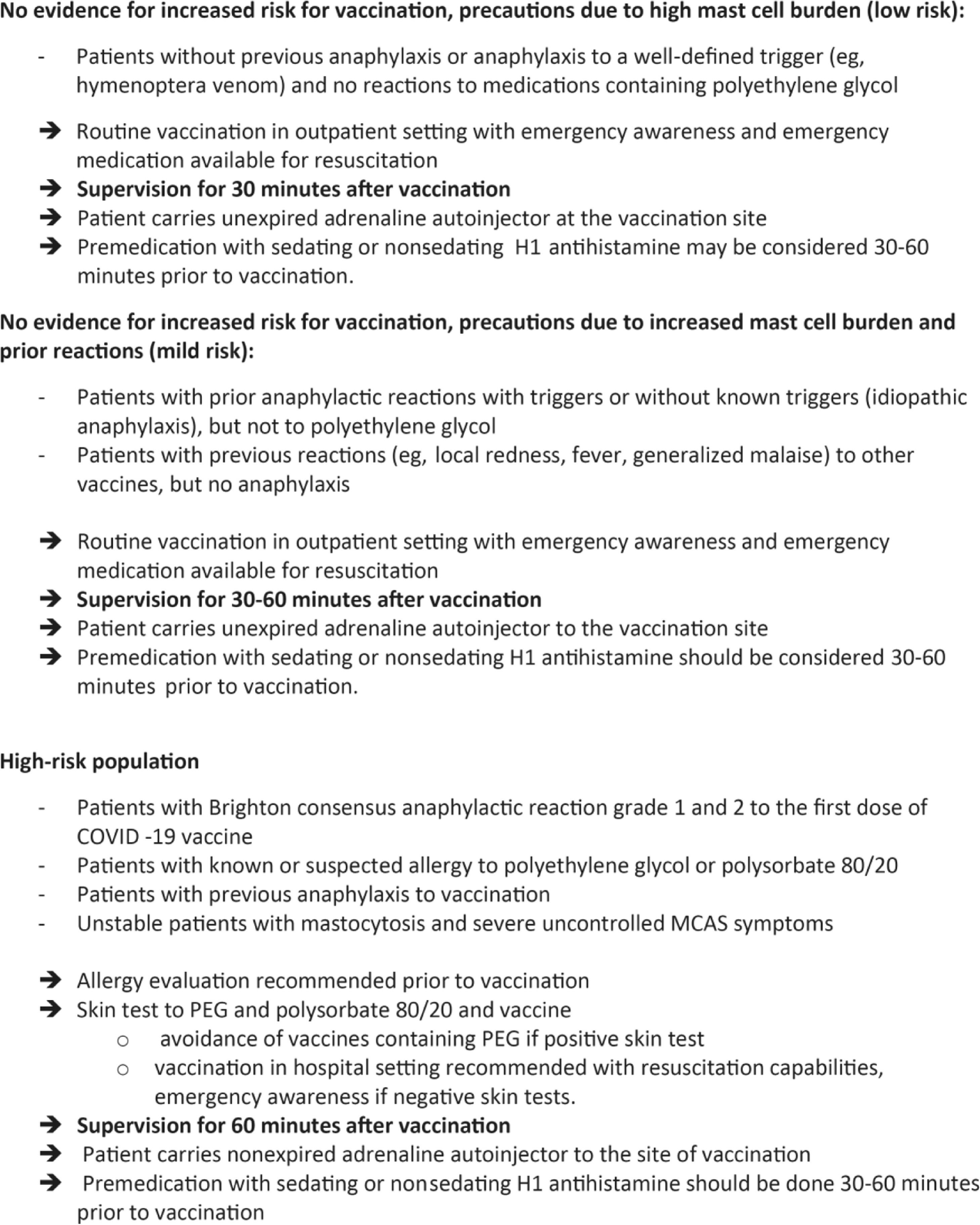
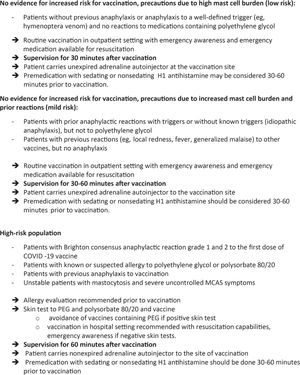
VaccinationThe European Competence Network on Mastocytosis and American Initiative in Mast Cell Diseases analyzed the risk-benefit ratio of the SARS-CoV-2 vaccination in patients with SM. It was concluded that there is no evidence that the incidence and severity of the reactions to the available vaccines are higher in patients with SM.101 Three risk categories were identified (Figure 2).
ECNM/AIMCD consensus guidelines100 for COVID-19 Vaccine Risk Stratification in Mastocytosis.*MCAS, mast cell activation syndrome; PEG, polyethylene glycol.*These recommendations are based on expert opinion and have not been evaluated regarding effectiveness.
A retrospective study102 of 35 children and 78 adult patients with mastocytosis analyzed clinical and vaccination records and found that children had a higher rate of local and/or systemic adverse reactions; all the cases were self-limiting and the patients recovered after symptomatic treatment. All the adults tolerated the vaccines without adverse events.
Recommendation: Vaccination, including the one against COVID-19, is indicated for all patients after prophylaxis with antihistamines on the same day (GRADE 4).
ConclusionsThe systemic mastocytosis comprises a group of mast-cell clonal diseases that range from indolent forms to more aggressive forms accompanied by hematological neoplasms, in which the gain-of-function KIT D186V mutation is the most frequent genomic finding. The disease is underdiagnosed and associated with high morbidity, including an increased risk of anaphylaxis and osteoporosis. Independent of the SM type, the symptom control is the mainstay of the therapy; the advanced forms should also receive cytoreductive therapy.
FundingFunding to support the expert meeting and the preparation of this manuscript was provided by Novartis. The authors take full responsibility for the content and conclusions stated in this manuscript.
Author contributionsEDRPV, GAP, AMMC, FPSS and PGB performed the literature research and prepared the questions for voting. All authors participated in the meeting and voting, reviewed the manuscript and approved the final version.
The authors would like to thank Ana Beatriz Studart (Department of Pathology at the HC-FMUSP), Luciana Nardinelli (Molecular Biology Laboratory of the Hematology Service at the HC-FMUSP) and Daniel Silva Nogueira (flow cytometry laboratory at the HC-FMUSP) for providing photographic documentation and Dr Mariangela Correa, MD, PhD for providing writing assistance on behalf of Springer Healthcare. The authors also express gratitude towards Novartis for the support of the mastocytosis research. This manuscript was prepared according to the International Society for Medical Publication Professionals–Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines.