Haploidentical stem cell transplantation (HSCT) is an option to treat patients with hemato-oncological diseases in countries where it is unlikely to find a matched related donor or a matched unrelated donor due to ethnic minorities.1 Considering HSCT as the curative option for these patients, the potential benefit of using post-transplant cyclophosphamide (PT-Cy) for graft-versus-host disease (GvHD) prophylaxis should be considered.2 However, regimen drug toxicity must be regarded during transplantation to identify patients at risk and anticipate probable complications.3 Cardiotoxicity, including coronary artery disease, heart failure, and cerebrovascular disease, is one of the most frequent adverse events related to anthracycline use. Furthermore, high doses of cyclophosphamide (Cy)4 can increase morbidity and mortality due to cardiotoxicity. However, other risk factors may increase the risk of cardiotoxicity in adults, such as a baseline cardiac heart compromise, history of arrhythmias, and low ejection fraction.3 The occurrence of cardiotoxicity in adults is approximately 0.9-5.5%,5 but the reports of post-transplant cardiotoxicity in pediatric patients are scarce, especially in patients with congenital heart disease (CHD) at baseline.
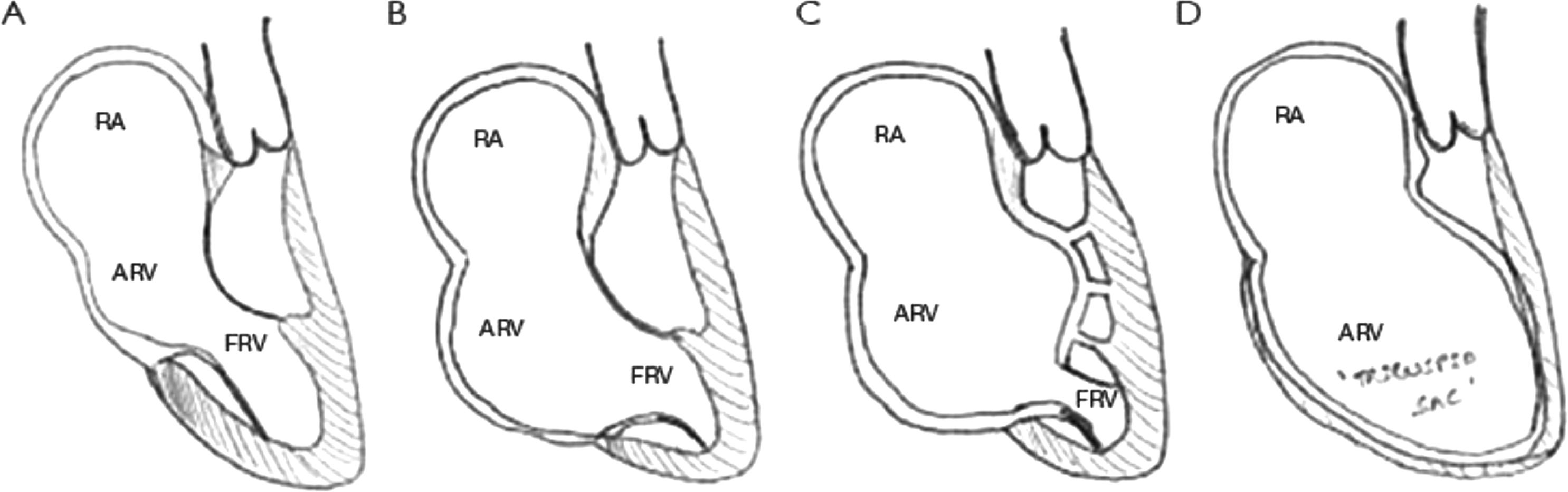
There are approximately 5-10 cases of CHD per 1000 live newborns. The Ebstein anomaly (EA) is a rare complex congenital malformation of the tricuspid valve with myopathy of the right ventricle.6 EA corresponds to <1% of all CHD7 and presents with variable clinical scenarios, varies from an incidental heart murmur or arrhythmias in children and adults to a severe symptomatic patient during the neonatal period.7 According to the volume of the functional right ventricle, this pathology is classified into 4 Carpentier´s categories (Figure 1). In type C, the anterior leaflet of the tricuspid valve is severely restricted in its movement and may cause significant obstruction of the right ventricular outflow tract with a small functional right ventricle.7 Medical management and observation is recommended for asymptomatic patients and may be successful for many years. For symptomatic patients, timely management with supplementary oxygen and prostaglandins or ECMO support should be made. In case of failure, a biventricular surgical repair with valvuloplasty or even heart transplantation is necessary due to the unstable hemodynamic condition with severe hypoxia. Mortality is related to presentation age and the severity of hemodynamic compromise.7 However, the overall survival in unoperated younger adults at one year and 20 years in 89% and 41% respectively.
Carpentier classification of Ebstein Anomaly. RA, right atrial; ARV, atrialized right ventricle; FRV, functional right ventricle. Copyright © from Journal of Thoracic Disease, Vol 12, No 3 March 2020.7
Multiple congenital syndromes that associated CHD with bone marrow dysfunction make stem cell transplantation a necessary procedure.5 Possibly the significant risks for post-transplanted children are endocarditis, structural heart defects repaired with foreign materials, and cardiotoxicity due to high doses of Cy. Nonetheless, the lack of information implies a need for further research of organ complications after HSCT in patients with multiple underlying diseases. In this case report, the objective is to show evidence in a real clinical scenario of a successful case of a patient with CHD who had a HSCT and received high dose Cy.
Case reportA thirteen-year-old female patient with Carpentier type C EA and high-risk precursor B-cell lymphoblastic leukemia was diagnosed around the time of her eighth birthday. She received extra-institutional complete anthracycline-free COG chemotherapy with a good response. Two years and five months after the last treatment, she had a marrow relapse with 93% lymphoid blasts for which she received rescue therapy based on vincristine/prednisolone/doxorubicin/asparaginase, with 24% blasts in the bone marrow at the end of induction. Second-line chemotherapy with clofarabine/cyclophosphamide/etoposide was given, and minimal residual disease was negative at the end of induction. Therefore, the medical group decided to perform an allogeneic transplant immediately. An HLA study found only haploidentical potential donors. Her father was 40 years old, was an HLA match of 5/10, had type 0+ blood, and was positive for cytomegalovirus (CMV) and toxoplasma IgG; he was selected as a donor to consolidate with HSCT. A pre-transplant echocardiogram showed moderate-to-severe tricuspid regurgitation, moderate dilation of the right ventricle with adequate systolic function, and mild mitral regurgitation without contraindications for HSCT.
Pre-transplant studies performed with serologies showed a positive CMV IgG receptor and negative residual bone marrow. Conditioning was performed with 40 mg/m2 of fludarabine from Days -6 to -3, 3.2 mg/m2 of intravenous busulfan on Days -6 and -5, TBI radiotherapy (400 CGY in two doses) on Day -2 and 2.5 mg/kg of thymoglobulin on Day -1. Thereafter, an infusion of hematopoietic progenitor cells was performed (source: bone marrow, CN: 26 × 108/kg, CD34 cells: 5603 × 106/kg, CD3 cells: 105 × 106/kg) without complications.
Post-transplant prophylaxis for GvHD started with 50 mg/kg of PT-Cy on Days +3 and +4, 5 mg/m2 of methotrexate (MTX) on Days +5, +7, +10, +15, with a rescue with calcium folinate at 24 h for each dose of MTX and cyclosporine beginning on Day +5 to maintain levels between 250 and 300 mcg/ml; antimicrobial prophylaxis was managed with acyclovir, posaconazole, and ciprofloxacin following institutional guidelines; and prevention of hepatic venous-occlusive disease (VOD) was prevented with ursodeoxycholic acid. On Day ten post-transplantation, she had a urinary infection caused by Escherichia coli and received management with cefepime. In addition, she presented with diarrhea with a presumptive diagnosis of grade II gastrointestinal GVHD due to the clinical presentation and timeline (14 days post-HSCT), with a good response to systemic and oral steroids. She had a CMV infection without target organ involvement 16 days after undergoing HSCT, for which she received ganciclovir treatment with good evolution. Neutrophil and platelet engraftment was evidenced at Day +16. In addition, on the day of the HSCT, the hemoglobin level was 12.5 mg/dL, the minimum level was 10.6 mg/dL at Day +12 post-transplant, and the maximum level was 17.9 mg/dL at Day +30.
Discharge occurred 31 days post-transplant with good evolution followed by the donor lymphocyte infusion (DLI) strategy based on chimerism. She reached 100% whole blood chimerism at Day +40, and post-HSCT bone marrow had minimal negative residual at Day +43. Afterward, she had mixed whole blood chimerism on two occasions: on Day +210 and Day +270. Therefore, we decided to suspend immunosuppression and treat using the DLI strategy on Day +240 using 25,000 CD3/kg, and on Day +270 using 50,000 CD3/kg. She had 100% recovery from chimerism, good evolution, and cardiac function unchanged. She did not have chronic GvHD (cGVHD).
One year after HSCT, progression of tricuspid insufficiency was observed in a follow-up echocardiogram. She started with progressive deterioration of functional class (NYHA II/IV), with increased cyanosis, dizziness, and polycythemia, requiring repeated phlebotomies. Four years after HSCT, at 17 years of age, the cardiology group decided to perform surgical management with a tricuspid commissurotomy; however, at the time of surgery, a deformed tricuspid valve was evidenced without a septal valve and a right ventricle without adequate musculature. Therefore, the procedure was suspended, and therapeutic cardiac catheterization was performed to embolize collateral vessels and try to improve symptoms.
Subsequently, she was taken to undergo a systemic pulmonary fistula and right ventricular plasty with the closure of the tricuspid orifice with an autologous pericardial patch and plication of the aneurysmal portion ventricle. During the postoperative period, she developed post-chest closure bleeding, acute kidney injury, and adrenal insufficiency, for which she received management in the pediatric cardiovascular intensive care unit. She had an excellent clinical course, with progressive improvement in oxygen saturation and decreased cyanosis and dyspnea; she was discharged 11 days after the intervention with enalapril, furosemide, and acetylsalicylic acid.
At eight years post-transplantation, she is asymptomatic from a cardiovascular point of view, has an NYHA functional class of I/IV, is in remission, and does not have chronic complications. Diagnostic cardiac catheterization was performed with evidence of signs of pulmonary hypertension, for which she was started on bosentan. She is currently 21 years old and leads a typical everyday professional life.
DiscussionThis case report describes the outcomes of a patient receiving PT-Cy based haploidentical HSCT with a history of LLA and EA. To the best of our knowledge, our case is the first report with these specific conditions in a living patient with good cardiac outcomes.
Stem cell transplantation in a patient with cyanogenic cardiopathy is challenging, given the risks and cardiotoxicities of conditioning and GVHD prophylaxis. It has been described in adults that high doses of Cy during conditioning can induce cardiac toxicity.8,9 It occurs more frequently with doses ≥ 180 mg/kg, and signs and symptoms of congestive heart failure occur within the initial 2-3 weeks after transplant.4 Nevertheless, there is little literature about cases similar to this in which high doses of PTCy for GVHD prophylaxis were used.
Even though cardiac toxicities have been described with PT-Cy in adults, this complication did not occur in our report. The use of lower doses of PT-Cy to prevent GVHD has recently been documented. Probably lower doses of PT-Cy should be used in patients with congenital heart disease, similar to those reported in non-neoplastic pathologies.10
Although the risk of post-HSCT cardiotoxicity using anthracyclines and Cy11,12 has been documented previously, there is still controversy. Some studies have mentioned that having a pre-transplant LVEF less than 45-50% does not interfere with transplantation performance and does not generate repercussions on survival.13 Similarly, the study by Lin et al. 14 mentioned that patients using PTCy did not have an increased risk of cardiotoxicity compared to patients who did not use PTCy.
Our case is relevant because the literature has shown two different results with the use of PTCy. In this case, we documented that in high-risk neoplasia with cyanogenic heart disease, HSCT with PTCy is a viable option in patients who do not have other therapeutic alternatives. We observed a long follow-up period in a living patient without hematological complications or cardiotoxicity.
In conclusion, congenital heart disease might not be an absolute contraindication for HSCT with PT-Cy in patients with no other therapeutic option.
Ethics approvalThe Institutional Ethics Committee approved the study following the Declaration of Helsinki. The participant gave informed consent before inclusion in the study.
This work was supported by Fundación Valle del Lili.