This study aims to evaluate the markers of tubular phosphate handling in adults with sickle cell anemia (SCA) and the influence of hydroxyurea (HU), the degree of anemia and Hb F concentration on these markers.
MethodsEighty-eight steady state SCA patients in outpatient follow-up in Fortaleza, Ceara, Brazil and 31 healthy individuals were included in this study. Vitamin D (25OHD) was measured by enzyme-bound fluorescence assay, intact parathyroid hormone (iPTH) by electrochemiluminescence, and serum and urinary phosphate and creatinine by colorimetric methods. Details of Hb F and HU use were obtained from clinical records. Tubular reabsorption of phosphate (TRP) and maximum tubular reabsorption of phosphate (MTRP) were calculated. SCA patients were stratified according to the use of HU, degree of anemia and percentage of Hb F. The significance level was set for p-values <0.05.
ResultsCompared to controls the 25OHD level (25 ± 11 vs. 30 ± 9 pg/mL) was lower in SCA, while serum phosphate and MTRP were higher (3.86 ± 0.94 vs. 3.46 ± 0.72 and 3.6 ± 1.21 vs. 3.21 ± 0.53, respectively). There was no significant difference in iPTH, TRP and phosphaturia. Serum phosphate showed correlation with TRP (r = 0.32; p-value = 0.008) and MTRP (r = 0.9; p-value <0.001) in SCA. Patients taking HU, especially those with Hb F >10 % presented reduced serum phosphate levels, and TRP and MTRP rates. Those with mild anemia presented reduced serum phosphate levels and MTRP rates.
ConclusionSerum phosphate levels and renal phosphate reabsorption rate were increased in SCA. HU use, high Hb F concentration and total Hb were associated with better control of tubular phosphate handling markers.
Sickle cell anemia (SCA) is an autosomal recessive disease caused by a point mutation in the β-globin gene, leading to the formation of hemoglobin S (Hb S) in homozygosis. At low pH or oxygen tensions, it polymerizes and precipitates resulting in vaso-occlusion and hemolysis.1,2 Chronic inflammation is induced with cell activation, and the production of pro-inflammatory cytokines and reactive oxygen species.3
This condition may lead to the development of sickle cell nephropathy, which is characterized by medullary hypoperfusion and regional hyperperfusion caused by reduced systemic vascular resistance, resulting in hematuria, tubular injury, hyposthenuria, glomerular injury with proteinuria and renal hypertrophy. This results in increased phosphate resorption and creatinine excretion in the proximal convoluted tubules (PCT). Intermittent episodes of vaso-occlusion favor the occurrence of acute renal injury, which may lead to the development of chronic kidney disease (CKD), an important factor in morbidity and mortality among individuals with SCA.4,5
HU is the main medicine used to control SCA because it induces the recruitment of erythroid precursors. The recruitment of erythroid precursors is responsible for increased Hb F production, reducing Hb S concentration in red blood cells and hindering sickling. It also reduces leukocyte, platelet and reticulocyte counts, modulating inflammation, endothelial damage, hemolysis and oxidative stress. HU is capable of reducing the incidence of complications associated with sickle cell nephropathy. In adults, six months of HU use reduced the incidence of albuminuria in patients who had presented with microalbuminuria.6,7
Chronically elevated free hemoglobin (Hb) concentrations in the lumen of PCT reduce the uptake of vitamin D by tubular cells and increase the transcription of 1-α hydroxylase messenger RNA, an enzyme responsible for vitamin D activation in the kidneys. In a SCA murine model, there is a decrease in the 1,25-dihydroxyvitamin D/25-hydroxyvitamin D ratio (1.25OHD/25OHD) indicating a reduction in circulating active vitamin D levels.8 Vitamin D deficiency and insufficiency, characterized by 25OHD levels below 20 ng/mL and between 20 and 30 ng/mL, respectively, are also common in SCA. 9,10 They occur as a consequence of high energy expenditure and inadequate nutrient absorption associated with the disease.11 Thus, the pathophysiology of SCA contributes to the occurrence of disturbances in the calcium and phosphate metabolism.
Phosphorus is an extremely important element in the maintenance of various cellular functions.12 The human body uses it in the form of phosphate (PO43−), which can be obtained from the diet in organic or inorganic forms. Serum levels of inorganic phosphate are influenced by the circadian rhythm, presenting the lowest values between 8 and 10 a.m. 13,14 They are regulated by the expression of the NaPi–IIa and -IIc cotransporters in the PCTs of the juxtamedullary nephrons and the cotransporter NaPi-IIb in the intestine according to the signaling from phosphate ion, iPTH and fibroblast growth factor 23 (FGF23).12
Markers used to monitor phosphate metabolism include iPTH, serum phosphate, 24-hour urine phosphate, tubular reabsorption of phosphate (TRP) and maximum tubular reabsorption of phosphate (MTRP). 15 Serum phosphate levels are very well controlled by increasing or reducing the expression of NaPi-II cotransporters according to serum ion concentration, resulting in changes in phosphaturia.12 In research settings, it is more difficult to obtain 24-hour urine samples for evaluation. Recent urine samples can be used, but there is no consensus in the literature regarding the correlation of the values obtained from this method with the values obtained in 24-hour urine.16 Thus, TRP and MTRP can be used to evaluate tubular phosphate handling.
Compared to 24-hour urine phosphate, TRP better estimates the kidneys’ ability to excrete phosphates which is altered by diet and age.17 In CKD, reduced levels (<85 %) can be used to indicate the need for clinical intervention to maintain phosphate homeostasis.15,17 MTRP is more related to the intrinsic ability of the kidneys to excrete phosphates. It represents the maximum renal capacity of phosphate reabsorption per volume of glomerular filtrate and corresponds to the theoretical lower limit of serum phosphate below which all phosphate is reabsorbed by the kidneys. It is correlated with Hb levels and is independent of phosphate from diet, bones and cells.15,17
Thus, this study aims to evaluate the markers of tubular phosphate handling in adults with SCA and the influence of hydroxyurea, the degree of anemia and Hb F concentration on these markers.
MethodsThis is a cross-sectional and analytical study involving 88 patients with SCA in outpatient follow-up at the Hematology and Hemotherapy Center of Ceara (HEMOCE) and the Walter Cantidio University Hospital (HUWC) and a control group of 31 blood donors in Fortaleza, Ceara, Brazil between February and October 2021.
The research was elaborated and executed in accordance with guidelines and regulatory standards of research involving human beings defined in the Declaration of Helsinki and was approved by the HUWC Ethics Committee (number 4.424.203). All participants signed an informed consent form. Patients with diagnosis of SCA confirmed by electrophoresis or HPLC and on steady state were included according to criteria described by Ballas et al.18
Five mL blood samples were obtained in tubes with ethylenediaminetetraacetic acid (EDTA) to the measure Hb concentration and in tubes with separator gel to measure 25-hydroxyvitamin D, iPTH, serum phosphate and creatinine. Exams performed near the time of blood collection were chosen. Ten mL samples of recent urine were obtained for urinary phosphate and creatinine dosing. 25OHD was evaluated by the enzyme-bound fluorescence assay (ELFA) method, iPTH was obtained by electrochemiluminescence, and the other markers were analyzed by colorimetric methods. Age, gender, Hb F levels, degree of anemia, duration of HU use were obtained from medical records and interviews. TRP and MTRP rates were calculated according to the formulas15,21:
SCA patients were stratified according to use of HU and compared with control for phosphaturia, serum phosphate, TRP and MTRP. Regarding the use of HU, only patients on stable doses were considered. Patients were also categorized according to the degree of anemia as moderate/severe and mild anemia, as previously described.18 The other categorization used was according to the percentage of Hb F as >10 % and ≤ 10 %.19,20
Statistical analysis was performed in Graphpad Prism version 6.0. Variables are presented as means, standard deviation, medians, absolute frequencies and percentages. The chi-square test was used to verify the existence of any interaction between qualitative variables. The normality of quantitative variables was verified using the Shapiro-Wilk test. Mann-Whitney test and Kruskal-Wallis test with Dunn's post-test were adopted to compare two and three groups, respectively. The correlation between the clinical markers was verified with the calculation of Spearman's rank correlation coefficient. The level of significance adopted was p-value <0.05.
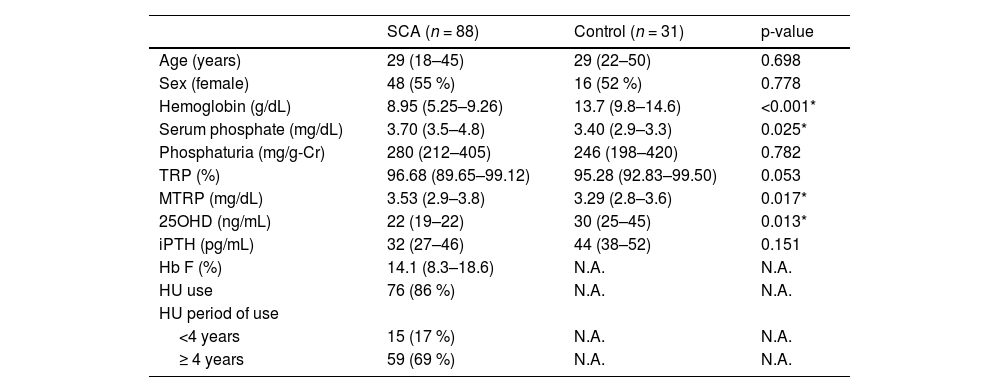
ResultsVariables related to phosphate metabolism are presented in Table 1. SCA patients had a mean age of 33 years, ranging from 18 to 74 years, and 55 % were female. Comparing SCA patients and controls, lower levels of total Hb (8.87 ± 1.72 g/dL vs. 13.86 ± 1.31 g/dL, p-value <0.001) and 25OHD (25 ± 11 ng/mL vs. 30 ± 9 ng/mL, p-value = 0.013) were observed in SCA, while serum phosphate and MTRP were slightly higher (3.86 ± 0.94 mg/dL vs. 3.46 ± 0.72 mg/dL; p-value = 0.025 and 3.6 ± 1.21 mg/dL vs. 3.21 ± 0.53 mg/dL; p-value = 0.017, respectively). There was no difference in iPTH, TRP and phosphaturia levels. Although SCA patients had lower 25OHD levels, only 30 (34.1 %) presented vitamin D deficiency (<20 ng/mL).
Demographic and laboratorial data for SCA and control groups.
Data presented as median and (interquartile range) or (absolute frequency - percentage). Mann-Whitney and chi-square tests applied. N.A.: Not applicable. *p-value <0.05. TRP: tubular reabsorption of phosphate; MTRP: maximum tubular reabsorption of phosphate; 25OHD: 25-hydroxyvitamin D; iPTH: intact parathyroid hormone; Hb F: fetal hemoglobin; HU: hydroxyurea.
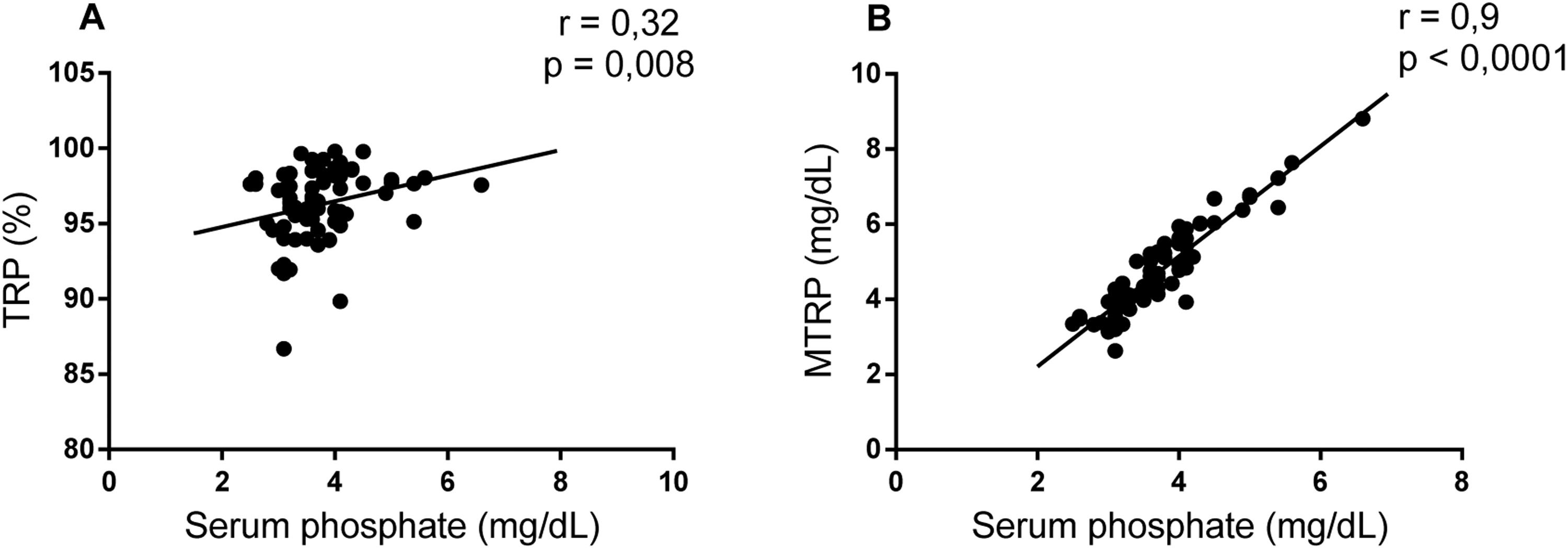
Considering the differences in markers associated with tubular phosphate handling between the groups, a correlation analysis was performed to understand whether these parameters were associated in SCA. Serum phosphate presented a weak correlation with RTP (r = 0.32; p-value = 0.008) and a strong association with RTMP (r = 0.9; p-value <0.001 - Figure 1).
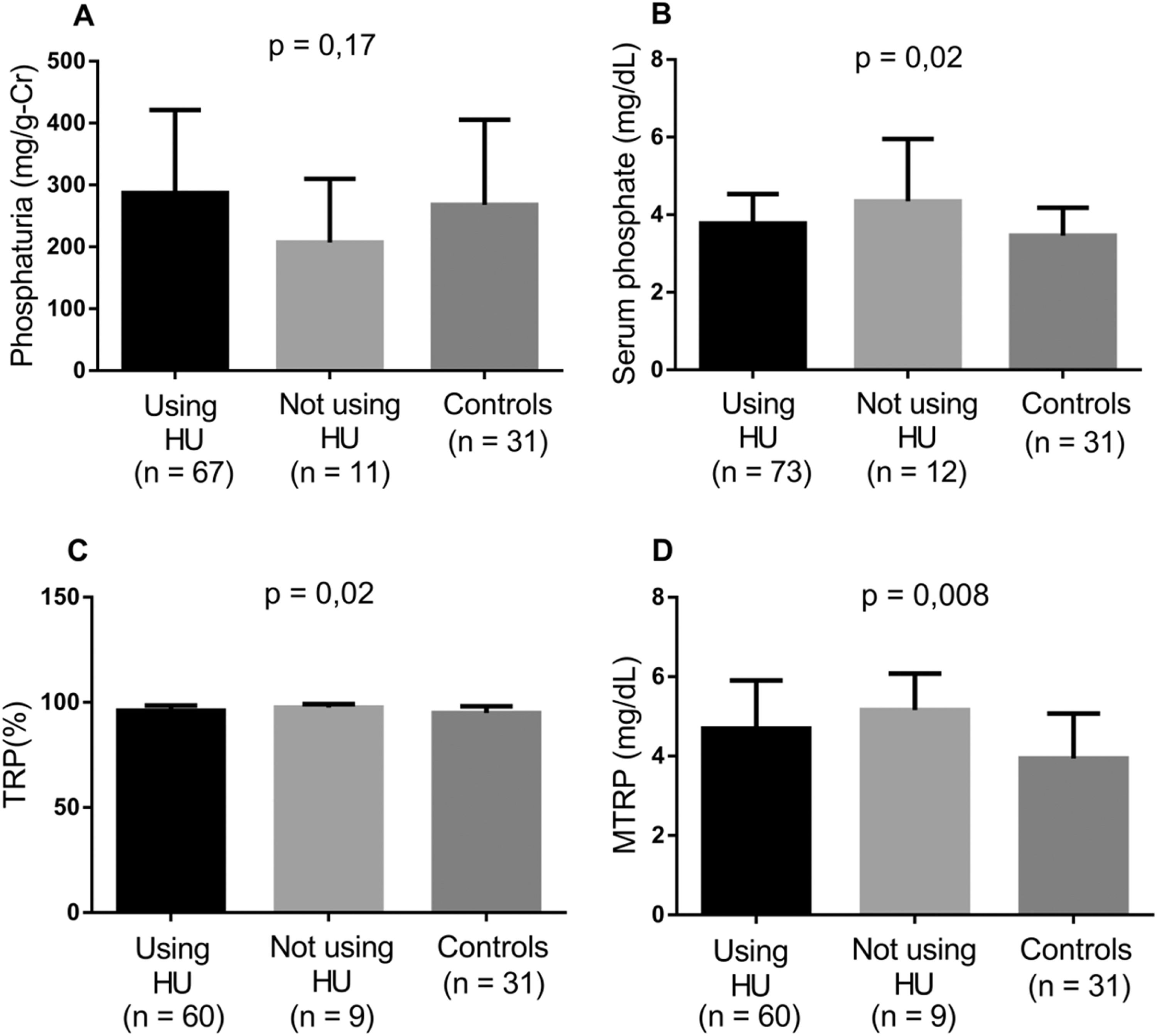
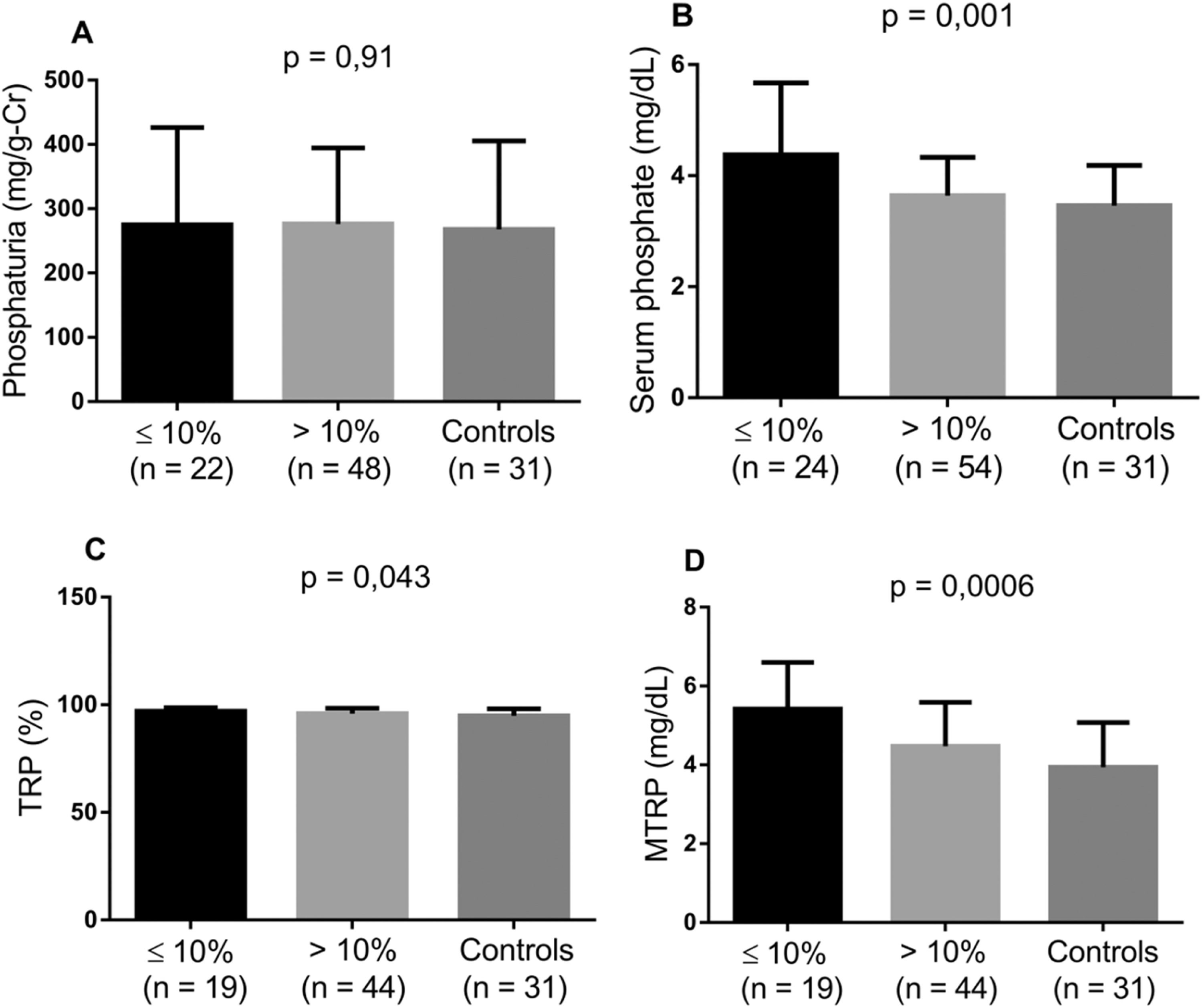
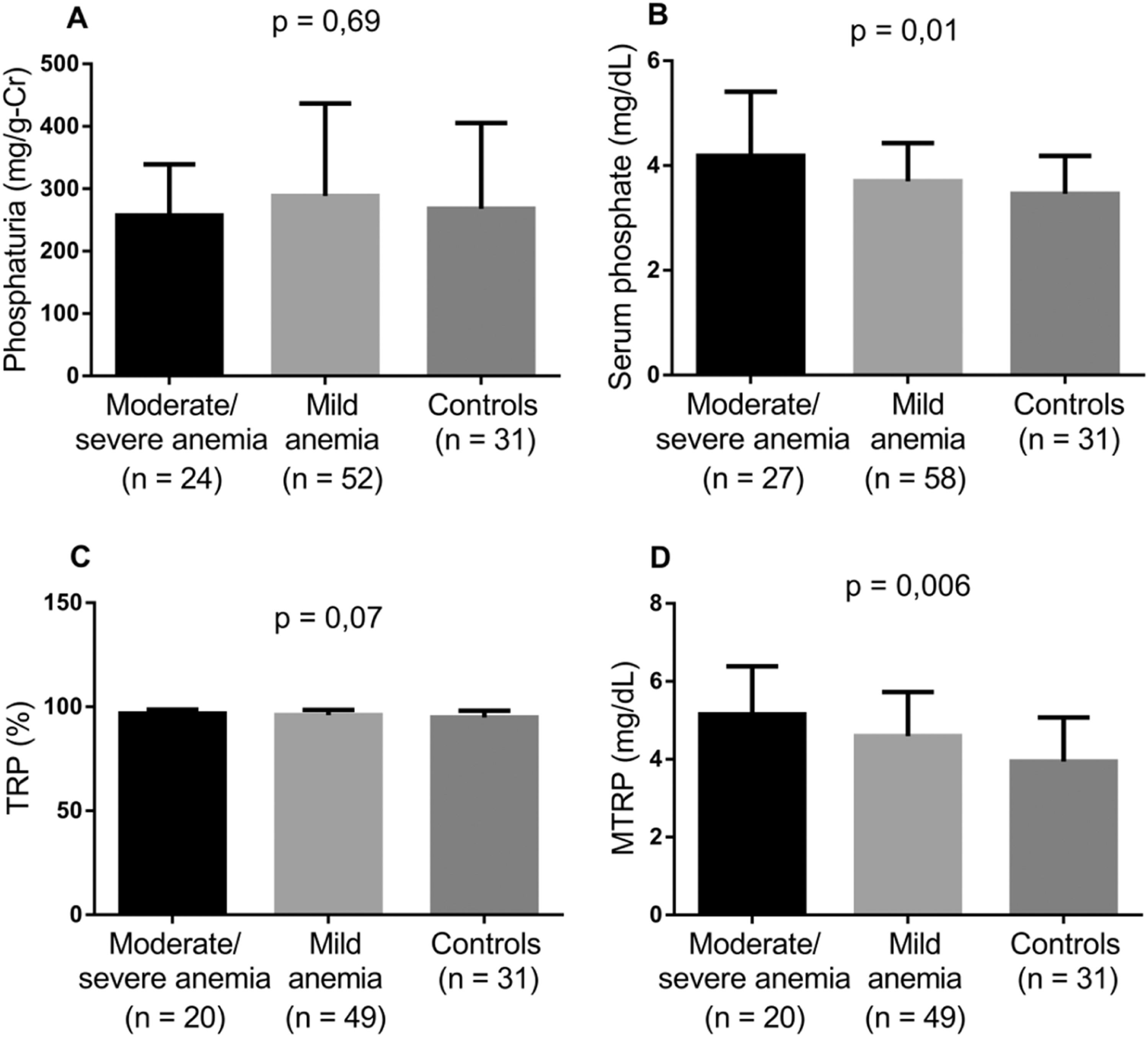
Considering the impact of higher Hb and Hb F values and the use of HU on the incidence of SCA complications, the markers involved in tubular phosphate handling were evaluated by comparing controls with the groups of patients stratified according to HU use, Hb F level and degree of anemia. Patients taking HU and those with Hb F >10 % presented lower levels of serum phosphate, TRP and MTRP (Figures 2 and 3). In individuals with mild anemia, there were lower levels of serum phosphate and MTRP (Figure 4).
Levels of biomarkers linked to phosphate metabolism comparing controls and two groups of SCA patients - those taking HU and those not (Kruskal-Wallis and Dunn's tests; p-value <0.05). A) Phosphaturia; B) Serum phosphate; C) Tubular reabsorption of phosphate (TRP); D) Maximum tubular reabsorption of phosphate (MTRP).
Levels of biomarkers linked to phosphate metabolism comparing controls and two groups of SCA patients - Hb F ≤ 10 % and Hb F >10 % (Kruskal-Wallis and Dunn's tests; p-value <0.05). A) Phosphaturia; B) Serum phosphate; C) Tubular reabsorption of phosphate (TRP); D) Maximum tubular reabsorption of phosphate (MTRP).
Levels of biomarkers linked to phosphate metabolism comparing controls and two groups of SCA patients – moderate/severe anemia and mild anemia (Kruskal-Wallis and Dunn's tests; p-value <0.05). A) Phosphaturia; B) Serum phosphate; C) Tubular reabsorption of phosphate (TRP); D) Maximum tubular reabsorption of phosphate (MTRP).
SCA presents diverse pathophysiological phenomena that affect multisystemic functions; in this context, the mineral and bone metabolism undergoes alteration of homeostasis due to the underlying disease. Vitamin D deficiency is a recurrent finding in studies with SCA patients, which usually report frequencies ranging from 56 to 96 %.10 This may be a consequence of reduced appetite and hypermetabolism induced by chronic inflammation and a high hemolysis rate. Vitamin D levels are also associated with age, diet, exposure to sunlight, skin pigmentation and kidney function.10,22 Vitamin D deficient adults with SCA are at higher risk of developing bone complications, such as osteomalacia, osteopenia and osteoporosis.23
The present study shows that serum phosphate levels were higher in SCA adults, although they were still within the reference range and samples were not collected from the control group during fasting because they were blood donors. There was no significant difference in phosphaturia or TRP between groups, which could be due to the small sample size. Nevertheless, TRP rates observed in controls may be an adaptation to phosphate consumption, as they tend to decrease to compensate for low dietary intake.15 However, there was no correlation between phosphaturia and TRP in any of the groups.
Elevated MTRP was present in SCA, evidencing a reduction in the intrinsic capacity of the kidneys to excrete phosphates. De Jong et al.24 and Smith et al.25, studying groups of 5 and 12 adult patients with SCA, respectively, described the occurrence of hyperphosphatemia associated with increased MTRP. This may be related to tubular injury, which is common in sickle cell nephropathy.4
The majority of patients in this study took HU, which may have impacted the profile observed. The high frequency of HU use may be due to the fact that recruitment for the study occurred during the COVID-19 pandemic, when the outpatient clinic began to offer teleconsultations. Thus, most of the time, only those who took HU and needed to renew their prescription for the drug went to that health clinic in person. In addition, most of the patients followed in this study presented with conditions that require the use of HU20, which is reflected in the predominance of long-term drug use.
There was a strong correlation between serum phosphate and MTRP, similar to that observed in children with SCA.21 The results obtained by Raj et al. suggest tubular resistance to the phosphaturic effect of FGF23, which was high in that population.
In SCA patients, HU use significantly alters tubular phosphate handling markers, contributing by increasing the capacity of renal phosphate excretion, intrinsically reduced in SCA, and reducing phosphatemia. Patients with Hb F >10 % also showed a reduction in these variables. In a study with the same group of SCA patients, Laurentino et al.26 found that HU use in doses above 10 mg/kg/day and for more than 50 months were associated to a reduction in the hemolysis rate. Moreira et al.20 found a decrease in hemolysis markers (lactate dehydrogenase level and reticulocyte count) in SCA patients with Hb F >10 %. Thus, it can be assumed that HU, by reducing hemolysis, helps normalize phosphate markers, potentially protecting from complications associated with changes in tubular phosphate handling in SCA.
Lower phosphatemia and MTRP values were observed in patients with Hb ≥8 g/dL. Serum phosphate levels are directly related to mild or moderate anemia even in patients with normal renal function or early-stage CKD. In individuals with GFR >60 mL/min/1.73 m2, high levels, but within the reference range, were associated with a higher risk of anemia.27 Tran et al.28 found that, with each 0.5 mg/dL elevation in serum phosphate, the chance of presenting moderate anemia increased by 16 %. Suggested explanations include phosphate increasing the production of uremic toxins, which inhibit erythropoiesis, and calcification of renal arteries resulting in erythropoietin deficiency.29,30 Along with other alterations inherent to SCA, these factors may contribute to the pathophysiology of the disease.
The main limitation of the present study is the limited sample size. However, it is important to emphasize that the patients included are treated at the main reference center in Hematology and Hematology in the state of Ceará, one of the largest in Brazil. Therefore, this convenience sample is representative of the local reality of sickle cell anemia.
ConclusionIn this study, increases in serum phosphate levels and renal phosphate reabsorption rate were observed in SCA. In addition, the use of HU and high Hb F and Hb values were associated with better control of serum phosphate, TRP and MTRP. Therefore, HU use can help to protect against complications associated with changes in phosphate handling in SCA patients.
This study was funded by Foundation Coordination for the Improvement of Higher Education Personnel (CAPES).