Although several combination therapies for acute myeloid leukemia (AML) have emerged recently, there has been a lack of published surveys and educational projects focused on these important treatment options. We aimed to improve the oncology team members’ knowledge and awareness of several FDA approved combination therapies for AML, including glasdegib (DAURISMO®), venetoclax (VENCLEXTA®), GO (MYOLOTARG®),CPX-351 (VYXEOS®), and midostaurin (RYDAPT®). Additionally, we aimed to examine these teams’ perspectives, views, and attitudes towards these topics and finally identify barriers to the implementationof such therapies in clinical practice.
MethodInitially, we developed booklets and then distributed them to each participating oncology and hematology office. Subsequently, all participating oncology and hematology team members were asked to complete an anonymous online survey to test their knowledge of and attitudes toward the subjects.
Main resultsThere was a total of 52 survey respondents. The correct answer regarding various combination therapies for AML was identified by nearly 70% or more of survey takers. The level of awareness of project subjects significantly improved after reading our printing materials. Many survey respondents were motivated to learn more about combination therapies for AML as well as discuss these topics with others.
ConclusionsOur booklets effectively improved understanding and awareness of combination therapies for AML. Future studies should explore awareness, knowledge, and perception of other new and emerging combination therapies for AML among oncology and hematology team members in other areas.
The American Cancer Society has predicted nearly 20,000 of new cases of AML and more than 11,000 deaths from this condition by the end of 2020.1,2 For nearly fifty years, most patients diagnosed with AML have been treated with the 7 days of continuous infusion of cytarabine and 3 days of anthracycline, also known as the ‘7+3’ regimen.1,2 The current remaining challenges in the treatment of AML are the improvement in overall survival and slowing of overall disease progression.1,2
There has been an improvement in our understanding of AML mechanisms, which resulted in several new therapies emerged and lately approved by the Federal Drug Administration (FDA).2,3 The benefits of some of these new therapies had particularly evident when these drugs were combined with the traditional ‘7+3’ approach.2,4 Some of the new medications, such as glasdegib, gemtuzumab ozogamicin (GO), or midostaurin, improved overall survival when combined with traditional standard therapies.5–7 CPX-351, which is a fixed-combination of daunorubicin and cytarabine, improved overall survival among patients with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC).5–7 Some newly-diagnosed patients with AML on venetoclax and azacitidine achieved a complete remission.5–7 Other vital benefits, such as a favorable benefit-to-risk profile and generally manageable toxicity, were observed in patients receiving both glasdegib and low-dose cytarabine.5–7
Previously we were able to improve oncology and hematology team members’ knowledge of biosimilars and various aspects related to biosimilars such as the approval process, safety, interchangeability, and the potential of biosimilars to enable optimal combination therapy for cancer.8 Our educational materials used in this project effectively improved knowledge and awareness of many important concepts regarding biosimilars.
We hypothesized that some oncology and hematology team members in Colorado might have a lack of knowledge and awareness of some combination therapies for AML. As such, we intended to improve such knowledge and awareness via our curriculum based educational initiative accompanied by follow up survey. In addition, we also aimed to assess these members’ perspectives, views, and attitudes toward the concept of combination therapy for AML as well as identify barriers for the implementationof such therapies in clinical practice.
Material and methodsWe aimed to complete 2 phases of sequential interventions which have been previously shown to result in a greater impact on knowledge and behavior.9 Initially, we prepared printing materials based on a thorough literature review. We discussed several recently approved by FDA combinations such as glasdegib (DAURISMO®), venetoclax (VENCLEXTA®), GO (MYOLOTARG®), CPX-351 (VYXEOS®), and midostaurin (RYDAPT®).10–14 The content of booklets was developed with the idea in mind that some oncology and hematology team members may have a relatively incomplete knowledge of these therapies.
Alongside, we prepared a survey using a popular online tool (Surveymonkey®, Palo Alto, CA, USA) which has become a popular one among some researchers.15–17 The survey contained 30questions which included general questions, questions testing general knowledge, and awareness of various combination therapies for AML, two open questions, and some agreement statements. Our survey was also prepared based on a thorough literature review. Several volunteers working in various health fields, as well as with little and no medical background, were invited to test our booklets and survey to ensure their comprehensibility. A 10-point scale measuring the awareness level regarding combination therapies for AML, and motivation and interest was implemented. On this scale, 1 indicated no awareness, motivation or interest, and 10 indicated strong awareness, motivation or interest.
There were 82 oncology and hematology office teams were contacted. All the offices selected from the Colorado Department of Health Care, Policy and Financing website (https://www.colorado.gov/hcpf), including the oncology/hematology teams, were designated physician offices. We aimed to target all team members, including oncologists/hematologists, oncology and hematology physician fellows, nurse practitioners, patient navigators, physician assistants, registered dieticians, nurses, and oncology and hematology surgeons. Each participating office received our package, which contained several educational booklets, a letter explaining the study as well as several gift cards to encourage survey participation. The survey was open for three consecutive months (between December 1, 2019 and February 28, 2020). We followed up with each participating office to encourage participation in our survey. All survey takers were asked to provide informed consent. Furthermore, no information such as an IP address that could potentially identify the respondent was collected. We informed all oncology and hematology team members that their responses would help us develop a research publication.
This study was determined as exempt by Integreview IRB on 11 October, 2019. The study was performed in accordance with the ethical standards of the Declaration of Helsinki. Prior to the beginning of the online survey, we provided an information sheet explaining the purpose of our study, how the data will be used, and stated that the data is anonymous.
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS®) software (version 16, SPSS®, Inc, Chicago, IL, USA). Due to a relatively small sample size, we combined participants’ responses into more meaningful categories (i.e., “strongly agree” and “agree”). A non-parametric Chi square test was used to analyze Likert scale data.18 P-values smaller than 0.05 were considered statistically significant. A one-sample Wilcoxon signed-rank test was applied for the study subject and the test criteria Z and p values were reported.
ResultsThere was a total of 52 survey respondents. One respondent did not agree to the informed consent, and the other did not answer to any questions other than from the informed consent. These two cases were deleted from our database. About one in eight (12%) of respondents were physicians (oncologists/hematologists), and more than 60% of survey takers were either nurses or nurse practitioners. Roughly one out of four (24%) were either patient navigators or belonged to the “other” category. Forty percent of respondents spent less than 5 years in practice; about one third (36%) spent 5 to 10 years, and approximately one in four did so for more than 10 years. About two thirds (66%) of survey takers were 21–40 years old, less than one third (30%) were 41–60 years old and 4% were 61–80 years old. More than three quarters (78%) of respondents were females.
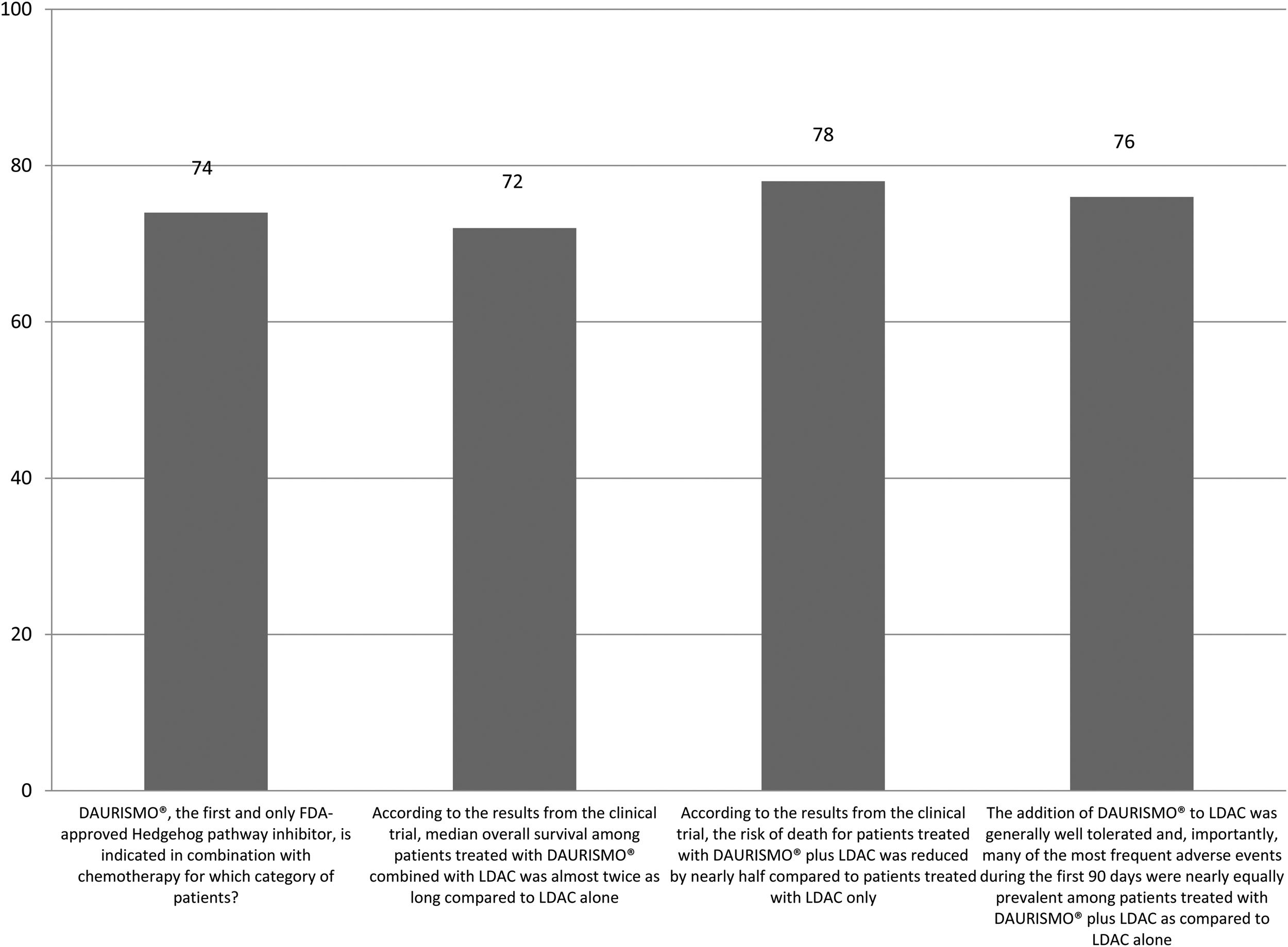
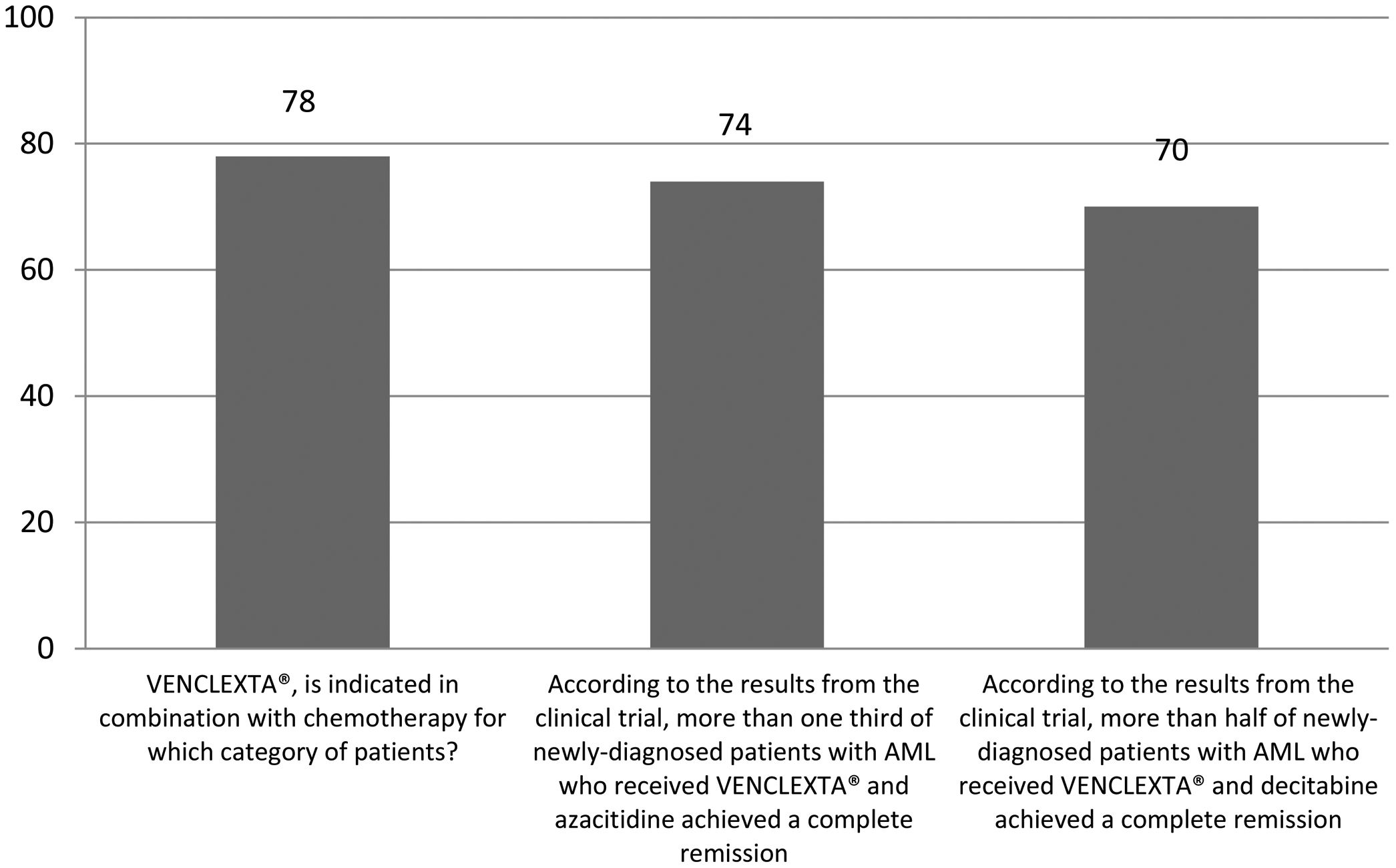
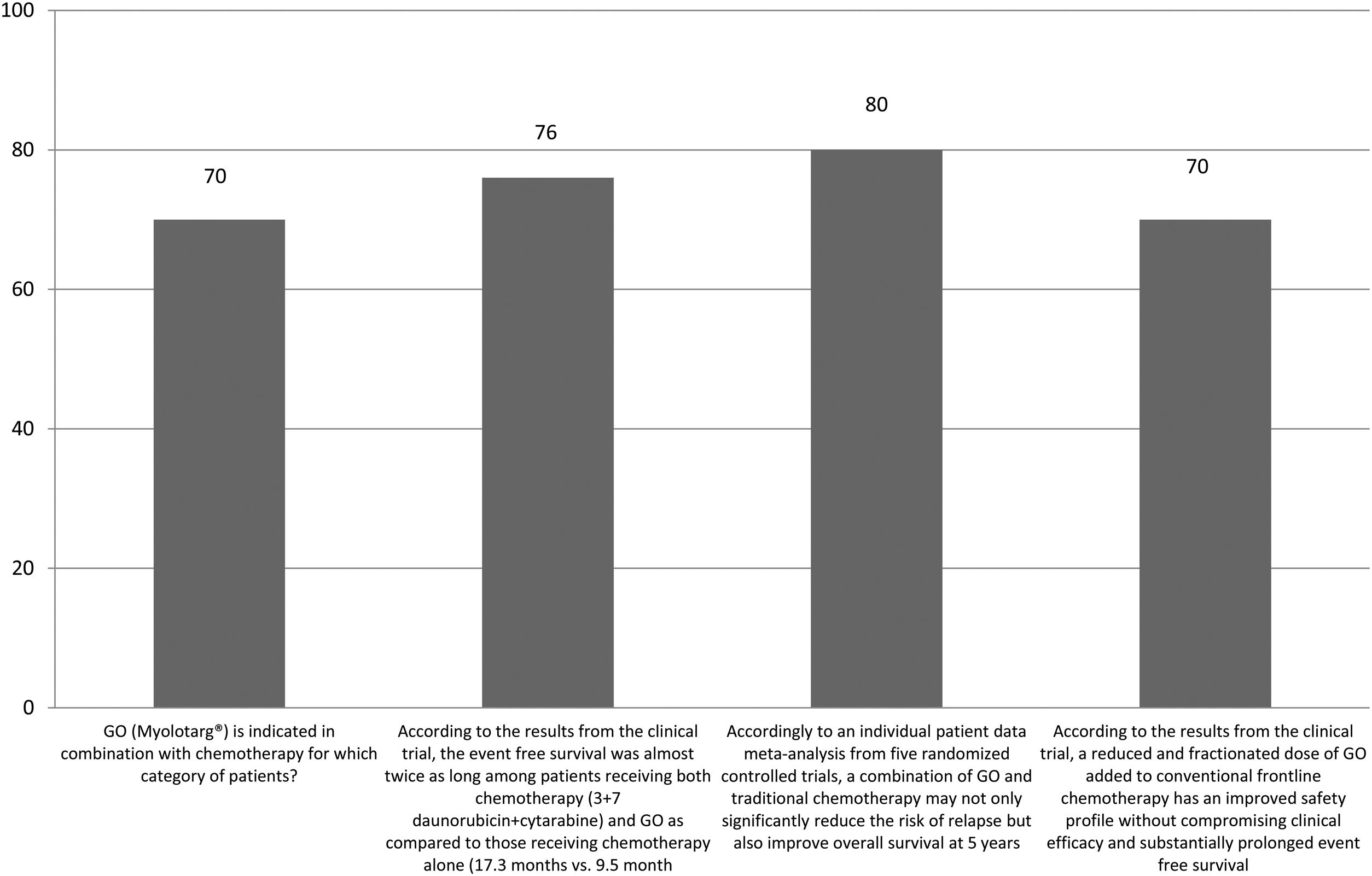
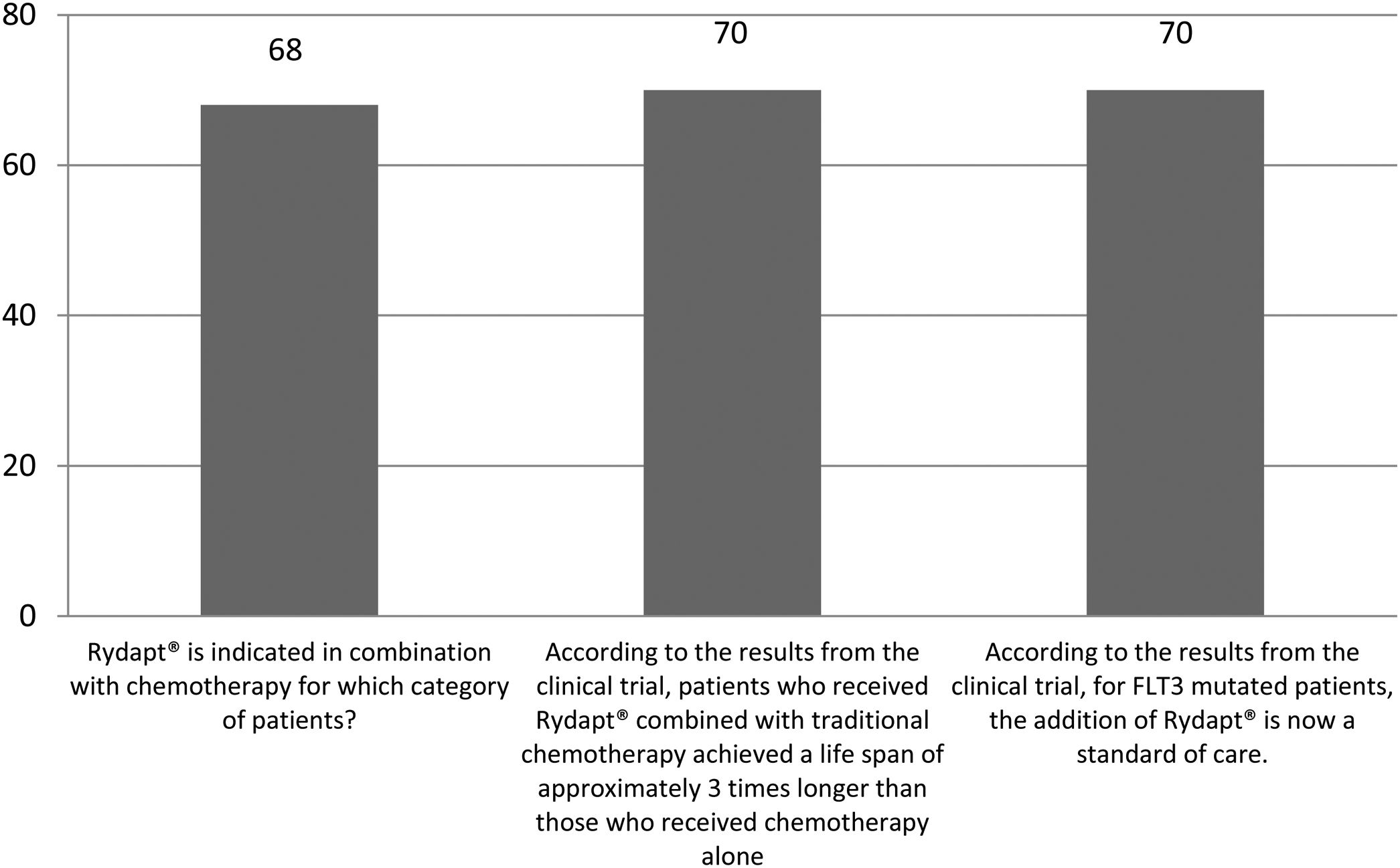
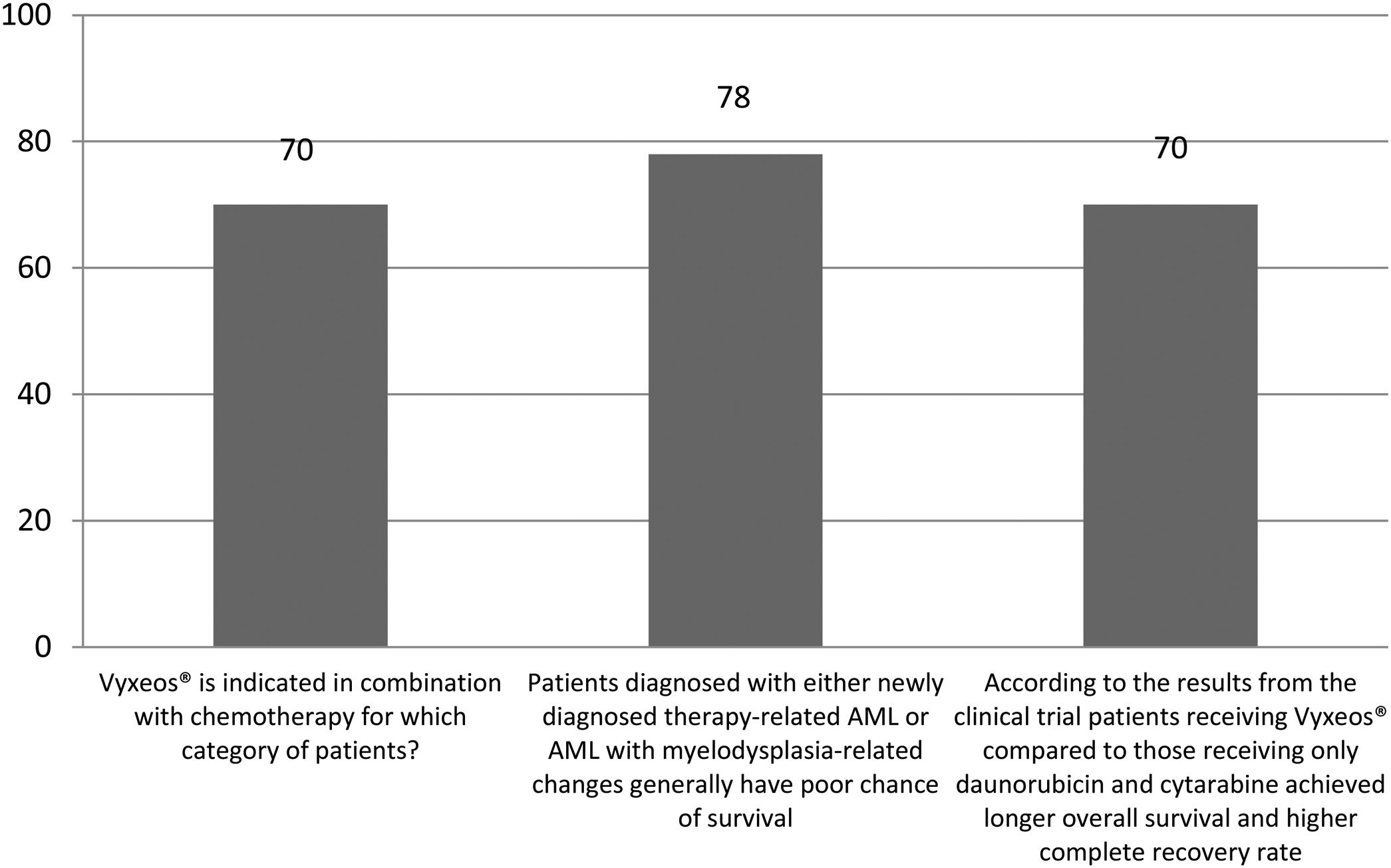
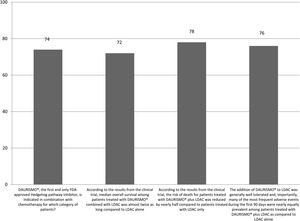
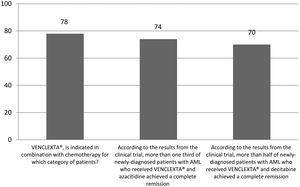
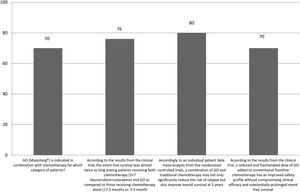
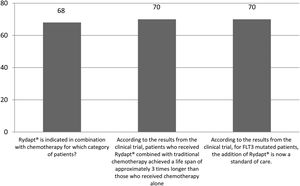
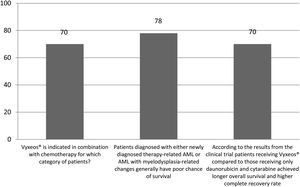
The correct answer regarding various combination therapies for AML was identified by approximately 70% or more of survey respondents (Figures 1–5). The correct answer to the question “Rydapt® is indicated in combination with chemotherapy for which category of patients?” was selected by 68% of survey takers. More than three quarters (78%) of respondents either agreed or strongly agreed that the landscape of AML treatment has been changed markedly through the introduction of new combination therapies approved by the FDA (Table 1). Of those, all oncologists/hematologists agreed to this statement, and majority of nurses/nurse practitioners (78%) either agreed or strongly agreed on this statement. Interestingly, the absolute majority of respondents (92%) who spent more than 10 years in practice and only two thirds (67%) of those who spent between 5 to 10 years agreed with the statement. The proportion of younger respondents who either agreed or strongly agreed with this statement was smaller (73%) while among those 41 to 60 years old, and 61 to 80 years old these proportions were 87% and 100% respectively.
Responses to the statement: “The landscape of AML treatment has been changed markedly through the introduction of new combination therapies approved by the FDA”.
| The degree of agreement | |||||
|---|---|---|---|---|---|
| Neither agree nor disagree | Agree | Strongly agree | Total | ||
| Oncology team members specialties | Oncologists | 0 | 6 | 0 | 6 |
| Nurse/nurse practitioner | 7 | 24 | 1 | 32 | |
| Patient navigator | 0 | 2 | 0 | 2 | |
| Other | 4 | 5 | 1 | 10 | |
| Years in practice | Less than 5 years | 5 | 14 | 1 | 20 |
| Between 5 to 10 years | 6 | 12 | 0 | 18 | |
| More than 10 years | 0 | 11 | 1 | 12 | |
| Age group | 21–40 years old | 9 | 24 | 0 | 33 |
| 41–60 years old | 2 | 11 | 2 | 15 | |
| 61–80 years old | 0 | 2 | 0 | 2 | |
There were 2 open questions in our survey. The first one was focused on the barriers to the use of combination therapies for AML and the other related to any topics concerning combination therapies for AML which are not well understood and for which directed education is needed. The first open question was answered by more than half (58%) of respondents. Of those, nearly half specified no barriers. The rest of the respondents provided answers related to either cost and price or lack of information. Similarly, the second open question was answered by slightly more than half (56%) of respondents of which 39% identified no topic of such concern. Other responses included “other” and “emerging” combination therapies for AML (18%), following by “insurance coverage” (7%), “safety” (7%), “long-term effects” (4%) and “specific indications and contraindications” (4%).
The level of awareness of combination therapies for AML prior to reading our booklets was poor (level 1 – 2) among 40% of survey respondents. Only about one in six (16%) of survey takers rated such level of awareness as “5” or “6”. After reading our booklets, about two thirds (66%) of survey respondents rated their level of awareness of the study subject as “6”, “7”,”8” and” 9”. Approximately one out of eight (14%) rated such awareness as “4”. Survey participants showed a significant improvement in scores after reading our booklets (z=6.018, p-value < 0.001).
Nearly 70% of respondents were motivated (motivation rank 6 – 10) to learn more about combination therapies for AML while about 30% were not (motivation rank 1 – 5, p-value < 0.05). Roughly three quarters (74%) were interested (interest rank 6 – 10) in discussing the study subject with others (e.g., medical professionals, family members, friends) while about a quarter (26%) were not (interest rank 1 – 5, p-value < 0.05). Printed materials such as brochures or flyers were the most popular style for learning more about combination therapies for AML (42%), followed by seminars and group discussions (30%), and finally webinars (24%). Two survey participants did not have any preference.
DiscussionAML is a relatively common condition that affects predominantly older adults.2,19 Once diagnosed, only one in ten older adults will live longer than 5 years.2,7 Moreover, one in three elderly patients does not receive active treatment as these patients are often diagnosed with various comorbidities making them ineligible for intensive chemotherapy.2,7 Emerging combination therapies for AML targeting these disadvantaged populations may improve the chances of survival and bring new hope.2,7,19 Additionally, new combination therapies may offer other important benefits, such as complete remission and improved event-free survival.2,7,19 As such, these emerging treatment approaches may open up new opportunities for oncology and hematology teams worldwide.
Several important themes emerge from our study results. We found that the baseline knowledge about combination therapies was relatively low. Fortunately, not only our educational materials effectively improved this, but our survey respondents were motivated to learn more about this subject in the future. Similarly, more than one-half of survey respondents in our previous study were very motivated to learn more regarding many important aspects related to biosimilars.8 Overall, the results from these studies may suggest that building an educational campaign to disseminate information focused on various important cancer treatment options could be effective among oncology and hematology team members. Our survey identified that younger and less experienced oncology and hematology team members were less likely to agree with important statements regarding combination therapies for AML, suggesting a significant need for more educational efforts targeting these groups.
According to our study results and possibly due to relatively recent approval of these treatment options, oncology and hematology team member’s baseline knowledge of these topics could be low. Our primary goal was to improve these teams’ knowledge and awareness of these very important subjects. Nevertheless, there could be a significant need for more educational efforts focused on this subject among other oncology and hematology teams.
In addition, we aimed to examine perspectives, views, and attitudes from oncology and hematology teams toward the concept of combination therapy for AML and identify barriers for the implementationof such therapies in clinical practice. Our study suggests that almost three quarters of participants were motivated to learn more about study subjects and discuss combination therapies for AML with others. We observed a somewhat larger proportion of motivated and interested oncology and hematology team members in our previous study focused on knowledge and awareness of biosimilars.8 To the best of our knowledge, there is a lack of studies focused on this topic among oncology and hematology team members worldwide. As such, prospective studies should explore this potentially very important subject among oncology and hematology team members from other geographic areas.
Many of our participating nurses and other staff members other than doctors became knowledgeable regarding study subject. Although the aim of oncology nursing is to effectively care for patients diagnosed with AML, however, oncology nurses as well as other oncology and hematology team members should be viewed in many other important roles such as patient education. As overall treatment for AML may last for many months, such long term relationships between the nurse and AML patient may become ultimate source of information regarding various other treatment options (i.e., combination therapies) beyond the immediate scope of the treatment plan.
Some of our respondents were concerned about the safety as well as contraindications of combination therapy for AML. Although the goal of these therapies is to use lower doses, avoid adverse effects and minimize expected side effects, some participants may still have a lack of awareness of these issues. As such, future educational and research studies should not only address these concerns but also focus on other related topics such as optimized protocols as well as long-term effect data for newer combination therapies for AML.
Overall, there is a lack of published surveys and educational projects focused on combination therapies for AML. There has been a survey of 2,621 hematologists in Brazil conducted between March and May of 2018.19 The incorporation of emerging therapies for AML was highly expected in that study, with more than 60% of survey respondents having expected midostaurin and more than half doing so for GO.19
Our study had some strengths and limitations. Unlike in a clinical trial, we collected our data from a real-world population and from various team members such as oncologists or oncology nurses. As mentioned earlier, there has been a paucity of published literature focused on various combination therapies for AML among oncology and hematology team members. To the best of our knowledge, this was the first study focused on improving knowledge and awareness of the study subjects among oncology and hematology team members and this was our strength as well. Finally, it was also the first attempt at assessing the oncology and hematology team members’ interest in sharing knowledge concerning combination therapies for AML with their colleagues and at evaluating oncology and hematology team members’ overall interest in the subject as well as motivation to complete additional training(s) focused on the subject in the future.
Our study also had some limitations. Due to the pilot nature of this project, only few combinations therapies for AML were discussed. In addition, our study results indicate that some oncology and hematology team members had a poor baseline awareness of combination therapies for AML. It could be that less knowledgeable team members participated in our educational activity and survey. Although many survey respondents were motivated to learn more about the study subject, it could be that more motivated providers responded to the survey. Unfortunately, we don’t have information if less motivated oncology and hematology team members agreed with our statements regarding combination therapies for AML. We also don’t know if less motivated members had a better or worse awareness of the project topics. Finally, many of our participants preferred printing materials to learn more about the subject but it is possible that oncology and hematology team members that favored seminars or webinars did not participate.
Our response rate is less than one per participating office and we don’t have information whether non-respondents had a better or worse knowledge of combination therapies for AML. We believe that both motivation and response rate will improve as more research studies focused on combination therapies for AML and which show promising results and favorable safety profiles will emerge.
ConclusionsThe baseline knowledge and awareness of emerging combination therapies for AML among oncology and hematology team members was relatively low. We improved such knowledge through our effective educational program, which was based on our printed materials. Similar programs should target younger and less experienced oncology and hematology team members in the future. Prospective studies should not only use our strengths and limitations but also develop curriculum-based initiatives that will discuss other emerging combination therapies for AML. Such studies should include follow up surveys to test knowledge and awareness of combination therapies for AML before and after exposure to the educational module (i.e., printed or online materials). Since AML continues to be a significant concern throughout the world, there is a great need for future educational projects focused on these therapies in various clinical settings.
FundingThis study was supported by Pfizer (grant number 53900193) and by the CMDAT Research Foundation. Pfizer was not involved in the design and conduct of the study, selection of investigators as well as data collection and management.
Conflicts of interestDr. Rovshan M Ismailov received financial support for educational programs from Pfizer, Amgen, Abbvie, Genentech, Novartis, Santen and Actelion. Dr. Dyana Saenz declares that she has no conflicts of interest. Dr. Pere Gascon received lecture fees from Sandoz, Amgen, Pfizer. Dr. Marcio Nucci received speaker fees from Pfizer, MSD, Basilea, Astellas, Abbvie, Janssen, Amgen, United Medical and Biotoscana; consultancy fees from Pfizer, MSD, F2G, United Medical and Biotoscana. Dr. Zaytuna Khasanova declares that she has no conflicts of interest.