High-dose cytarabine is considered standard of care as consolidation chemotherapy in adults with acute myeloid leukemia (AML) who are not eligible for allogeneic hematopoietic cell transplantation, but may be associated with significant toxicity. We evaluated the toxicity associated with high-dose cytarabine given as consolidation in AML patients treated at a Brazilian public hospital.
MethodsWe retrospectively reviewed the charts of all patients with AML treated between 2008 and 2020 who obtained complete remission (CR) after one cycle of induction chemotherapy and received consolidation with at least one cycle of high-dose cytarabine (defined as 3 g/m2 every 12 h days 1, 3 and 5).
ResultsAmong 61 patients who received induction remission, 32 obtained CR and 28 received at least one cycle of high-dose cytarabine, for a total of 67 cycles (median 2 cycles per patient, range 1 – 4). In 45 cycles (67.2%) the patient was discharged after the end of chemotherapy, with a median of 6 days at home (range 3 – 8). Readmission occurred in 31 of the 45 cycles (68.9%). The most frequent toxicities were febrile neutropenia (56.7%), nausea and vomiting (23.9%), oral mucositis (14.9%) and diarrhea (11.9%). Bacteremia was documented in 13 cycles (34.2%). There were three cases of typhlitis and two of invasive fungal disease (aspergillosis and candidemia). Four patients died (14.3%), with two deaths considered treatment-related (candidemia and typhlitis).
ConclusionIn the setting of a Brazilian public hospital, high-dose cytarabine as consolidation therapy is feasible, with manageable toxicity profile.
Cytarabine is a backbone in the treatment of acute myeloid leukemia (AML). Patients fit to receive intensive chemotherapy usually receive the 7 + 3 induction regimen (cytarabine given by continuous infusion for 7 days and an anthracycline for 3 days).1 After achieving complete remission (CR), patients receive consolidation therapy with high-dose cytarabine. A landmark study published in 1994 compared cytarabine 100 mg/m2 for 5 days by continuous infusion with 400 mg/m2 for 5 days by continuous infusion and 3 g/m2 in a 3-hour infusion every 12 h on days 1, 3 and 5 (18 g/ m2), and established the high-dose regimen as standard post-remission treatment for AML patients who did not have a donor for allogeneic transplant.2
The regimen of high-dose cytarabine may be associated with significant toxicity that includes myelosuppression, oral and gastrointestinal mucositis, fever and neurologic toxicity among others.3-8 Of particular interest is the occurrence of prolonged and severe neutropenia and mucositis, which increase the risk for bacterial and fungal infection.9-11 In Brazil, about 70% of hematologists treating patients with AML use the regimen of high-dose cytarabine as consolidation, as reported in a survey.12 However, little is known about the toxicity associated with this regimen in the region. In this study, we sought to evaluate the toxicity associated with high-dose cytarabine given as consolidation in AML patients treated at a Brazilian public hospital.
Patients and methodsThis is a retrospective single-center study conducted at Hospital Universitário Clementino Fraga Filho, a tertiary university-affiliated public hospital located in the city of Rio de Janeiro, Brazil. The Hematology unit has 8 single-bed rooms with high efficiency particulate air (HEPA) filter and positive pressure, and five double-bed rooms without HEPA filter. The study was approved by the institution's Ethical Committee (“Comitê de Ética em Pesquisa do Hospital Universitário Clementino Fraga Filho”).
Patients were identified from the hospital medical records and the Hematology Service registry. We selected all patients with AML treated between 2008 and 2020 who obtained CR after one cycle of induction chemotherapy and received consolidation with at least 1 cycle of high-dose cytarabine, defined as a dose of 3 g/m2 every 12 h in a 3-hour infusion, given on days 1, 3 and 5 (total of 18 g/m2 per cycle). Allogeneic hematopoietic cell transplantation (HCT) was offered to patients with intermediate or high-risk AML who had a suitable donor. However, if allogeneic HCT was planned, patients received at least one cycle of high-dose cytarabine before transplant. We excluded patients with acute promyelocytic leukemia, patients previously treated in other institutions, those receiving high-dose cytarabine after obtaining CR with more than one cycle of induction, those receiving lower doses of cytarabine (e.g., 1–1.5 g/m2/dose), or receiving high-dose cytarabine in second remission or relapse.
Patients were managed during the cycles as follows: (1) after the last dose of high-dose cytarabine, filgrastim was given until neutrophil recovery; (2) depending on the general clinical conditions and logistics, patients were discharged, followed in the outpatient unit with 2 visits per week, and instructed to return immediately to the hospital in case of fever; (3) quinolone prophylaxis was usually given after discharge and maintained until neutrophil recovery or until the occurrence of febrile neutropenia; (4) antifungal prophylaxis was given at the discretion of the attending physician except for patients with prior invasive fungal disease (IFD) caused by a mold. In such circumstances, secondary prophylaxis with anti-mold azole was mandatory.
Data were collected from patients’ electronic medical records, using two case report forms (CRF) and a dictionary of terms. The first CRF contained demographic and clinical data of each patient, basic information about the diagnosis of AML, risk stratification, date and regimen of induction remission and the total number of cycles of high-dose cytarabine given after CR. The second CRF contained detailed information of each cycle of high-dose cytarabine, including hospital discharge after chemotherapy, readmission, duration of hospitalization, duration of neutropenia, use of filgrastim, antimicrobial prophylaxis, the occurrence of febrile neutropenia, antibiotic therapy during febrile neutropenia, documentation of infection, use of non-prophylactic antifungal agents, mucositis, bleeding and the occurrence of gastrointestinal, renal, hepatic, cardiac and neurologic toxicity.
Neutropenia was defined as an absolute neutrophil count <500/mm3, and fever was defined as an axillary temperature >38 °C. The episodes of febrile neutropenia were classified as fever of unknown origin, bacteremia, microbiologically documented infection without bacteremia, or clinically documented infection, as previously defined.13 Toxicity of chemotherapy was assessed using the Common Toxicity Criteria.14 Risk category classification was performed using the 2022 European Leukemia Net recommendations for diagnosis and management of AML.15
Categorical variables were expressed as absolute numbers and percentage and were compared using Chi-square or Fisher's exact test, as appropriate. Continuous variables were summarized as medians and ranges. All tests were 2-sided, and P values <0.05 were considered statistically significant. Analyses were performed using SPSS 21.0 for Windows (IBM, Inc.).
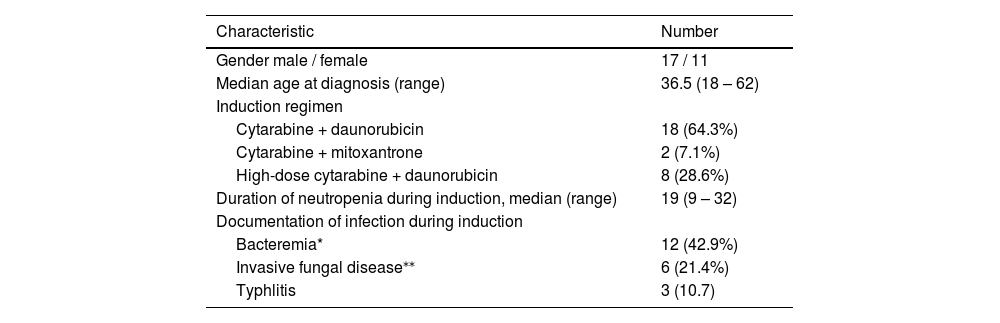
ResultsDuring the study period, 61 patients were diagnosed with AML and received induction remission. One patient died during induction and 32 (52.4%) obtained CR after one cycle of chemotherapy. Among the 32 patients who obtained CR, eight received other regimens of consolidation therapy and 28 received at least one cycle of cytarabine 3 g/m2 every 12 h on days 1, 3 and 5. The median age of these 28 patients was 36.5 years (range 18 – 72) and 39.3% were female.
Karyotype analysis was carried out in 24 patients (85.7%), and 10 presented abnormalities: t(8;21) in four, and trisomy of chromosome 22 (with t(16;16)), trisomy of chromosome 8, t(6;9), t(5;6), t(8;16), and complex karyotype (1 each). Molecular genetic testing was carried out in 18 patients (64.3%). NPM1 gene mutation was observed in three patients, FLT3 gene mutation in two cases, and RUNX1::RUNX1T1 in one. Among 13 patients in which we could classify risk category, six were classified in the favorable risk category, four in the intermediate group and three in the adverse risk group.
The most frequent regimen of induction was cytarabine plus daunorubicin (64.3%), followed by high-dose cytarabine plus daunorubicin (28.6%). The median duration of neutropenia in the induction phase was 19 days (range 9 – 32). Documentation of infection during induction was as follows: bacteremia in 12 (42.9%), IFD in six (21.4%) and typhlitis in three. Table 1 shows the characteristics of the patients.
Characteristics of 28 patients with acute myeloid leukemia who received high-dose cytarabine in consolidation.
| Characteristic | Number |
|---|---|
| Gender male / female | 17 / 11 |
| Median age at diagnosis (range) | 36.5 (18 – 62) |
| Induction regimen | |
| Cytarabine + daunorubicin | 18 (64.3%) |
| Cytarabine + mitoxantrone | 2 (7.1%) |
| High-dose cytarabine + daunorubicin | 8 (28.6%) |
| Duration of neutropenia during induction, median (range) | 19 (9 – 32) |
| Documentation of infection during induction | |
| Bacteremia* | 12 (42.9%) |
| Invasive fungal disease⁎⁎ | 6 (21.4%) |
| Typhlitis | 3 (10.7) |
A total of 67 cycles of high-dose cytarabine were given to the 28 patients, with a median of 2 cycles per patient (range 1 – 4). Nine patients received three cycles and four patients received four cycles. Filgrastim was given in 66 of the 67 cycles, usually from day 6 of the cycle until neutrophil recovery. In 45 cycles (67.2%) the patient was discharged after the end of chemotherapy, with a median of 6 days at home (range 3 – 8). Readmission occurred in 31 of the 45 cycles (68.9%). The median duration of neutropenia was 7 days (range 5 – 19).
Gastrointestinal toxicity was as follows: nausea and / or vomiting in 16 cycles (23.9%; grade 1–2 in 11, grade 3 in 5), oral mucositis in 10 cycles (14.9%; grade 1–2 in 9, grade 4 in 1), diarrhea in eight cycles (11.9%; grade 1 in 5, grade 2 in 1, grade 3 in 2). Two patients required parenteral nutrition. Neurologic toxicity was observed in two cycles (2 patients), consisting of seizure on day 2 of the cycle in one patient, and diplopia and dysmetria on day 10 of the cycle in the other patient. All neurologic abnormalities were reversible. Bleeding was observed in seven cycles (10.4%; grade 1 in 4, grade 2 in 2, grade 3 in 1).
Antibacterial prophylaxis with ciprofloxacin was given in 48 cycles (71.6%), including 40 of the 45 cycles in which patients were discharged after chemotherapy. Antifungal prophylaxis was given in 24 cycles (35.8%): fluconazole in 11 cycles, voriconazole in eight, itraconazole in three and posaconazole in one. All six patients with prior IFD caused by mold received anti-mold prophylaxis during consolidation.
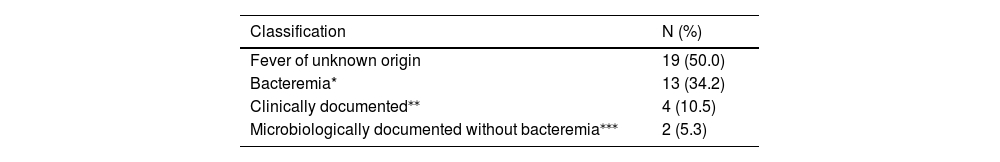
Febrile neutropenia was documented in 38 of the 67 cycles (56.7%). The frequency of febrile neutropenia was slightly higher in patients who remained in the hospital throughout the entire period of neutropenia compared with those who were discharged, but the difference was not statistically significant (68.2% vs. 51.1%, p = 0.19). Likewise, patients receiving quinolone prophylaxis were less likely to have febrile neutropenia (52.1% vs. 68.4% without quinolone prophylaxis), but the difference was not statistically significant (p = 0.17). The classification of the febrile episodes is shown in Table 2. Bacteremia was documented in 13 cycles (34.2%), and viridans streptococci and Escherichia coli were the most frequent agents (4 episodes each). Two cases of IFD were diagnosed: candidemia and aspergillosis (with concomitant viridans Streptococcus bacteremia). The patient who developed candidemia was not on antifungal prophylaxis. None of the six patients with prior mold infection presented relapse of the IFD. Typhlitis was diagnosed in three cycles. Two of these patients required parenteral nutrition and were transferred to an intensive care unit.
Classification of the episodes of febrile neutropenia (n = 38) after high-dose cytarabine.
| Classification | N (%) |
|---|---|
| Fever of unknown origin | 19 (50.0) |
| Bacteremia* | 13 (34.2) |
| Clinically documented⁎⁎ | 4 (10.5) |
| Microbiologically documented without bacteremia⁎⁎⁎ | 2 (5.3) |
OBS: 1 case of invasive aspergillosis and viridans Streptococcus bacteremia; 3 cases of typhlitis (one with Clostridioides difficile colitis, one with bacteremia due to E. coli and one with bacteremia due to Acinetobacter lwoffii).
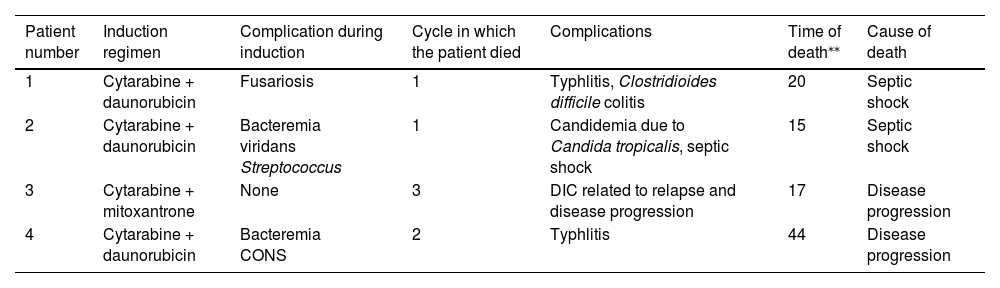
Overall, four patients died, with a death rate of 14.3% (6% of cycles). Two deaths were related to infection and considered treatment-related: septic shock with candidemia on the first day of febrile neutropenia, and sepsis with Clostridioides difficile colitis five days after neutrophil recovery. The other two deaths occurred in the context of relapse of AML and disease progression. Details of the four deaths are shown in Table 3.
Characteristics of four patients who died.
| Patient number | Induction regimen | Complication during induction | Cycle in which the patient died | Complications | Time of death⁎⁎ | Cause of death |
|---|---|---|---|---|---|---|
| 1 | Cytarabine + daunorubicin | Fusariosis | 1 | Typhlitis, Clostridioides difficile colitis | 20 | Septic shock |
| 2 | Cytarabine + daunorubicin | Bacteremia viridans Streptococcus | 1 | Candidemia due to Candida tropicalis, septic shock | 15 | Septic shock |
| 3 | Cytarabine + mitoxantrone | None | 3 | DIC related to relapse and disease progression | 17 | Disease progression |
| 4 | Cytarabine + daunorubicin | Bacteremia CONS | 2 | Typhlitis | 44 | Disease progression |
DIC = disseminated intravascular coagulation; CONS = coagulase-negative staphylococci.
In this retrospective study we observed that the median number of cycles of high-dose cytarabine per patient was two, and in most cycles, we were able to discharge patients at the end of chemotherapy, although in the majority of cycles the patient was readmitted because of febrile neutropenia. Febrile neutropenia, nausea and vomiting were frequent, and the rate of treatment-related mortality was low, with only two deaths attributed to toxicity (septic shock in the context of febrile neutropenia). Of note, these deaths occurred in the first consolidation cycle.
Cytarabine constitutes an indispensable component of AML consolidation, but it is associated with non-hematologic adverse events, mainly dose-dependent neurologic and gastrointestinal toxicities. The European Leukemia Net 2022 guidelines do not recommend high-dose cytarabine because of the high rates of toxicity compared with regiments with lower doses of cytarabine, and because no clear benefit was observed in terms of overall survival.15 Indeed, in a meta-analysis of nine trials where high-dose and standard-dose cytarabine were compared, high-dose cytarabine improved relapse-free survival but had no impact on overall survival.16 In another meta-analysis evaluating more than 4000 patients, high-dose cytarabine improved disease free survival compared with an intermediate dose. The authors concluded that cytarabine at a dose of 3 g/m2 twice daily provides maximal anti-relapse effect.17
More recently, new drugs have been approved for the treatment AML, and it remains to be established how to combine these new drugs with standard post-induction chemotherapy. In the RATIFY trial, patients were randomized to receive midostaurin or placebo just after standard induction with cytarabine and daunorubicin (“7 + 3”), and after obtaining CR, four cycles of high-dose cytarabine were planned together with midostaurin or placebo.18 Other trials testing quizartinib and sorafenib also used high-dose cytarabine (3 g/m2) in the consolidation phase.19,20
Another unaddressed question regarding consolidation with cytarabine is the optimal number of cycles. In our study, only 14% of patients received four consolidation cycles. This rate was inferior to the original CALGB trial, where more than 50% of patients received the four planned consolidation cycles.2 The AML17 trial compared one to two high-dose cytarabine courses in patients who obtained CR with two induction chemotherapy courses. The addition of the second course of high-dose cytarabine improved relapse-free survival but did not have any impact on the overall survival.21
In our study, documentation of infection was observed in 50% of the episodes of febrile neutropenia, with 34.2% of bacteremia. Typhlitis was diagnosed in three cycles and IFD in two. The incidence of typhlitis in patients with AML varies widely, depending on the chemotherapeutic regimen, with higher incidence rates in patients receiving drugs that cause severe mucositis.22 Among the two cases of IFD, one patient developed candidemia due to Candida tropicalis, with septic shock and death. Of note, the patient was not receiving antifungal prophylaxis. Fluconazole prophylaxis is associated with a reduction in the incidence of candidemia in patients with AML receiving induction remission chemotherapy.23 However, its role in consolidation therapy is controversial, considering that the overwhelming majority of cases of IFD in patients with AML occur during induction and not after consolidation.24
Several factors are likely to reduce treatment costs in AML, including early hospital discharge and outpatient treatment. In our study, early hospital discharge was possible in 67% of cycles. However, in almost 70% of cycles in which the patient was discharged after chemotherapy, readmission was necessary. In another single-center retrospective study conducted at a Brazilian hospital, 27 AML patients received high-dose cytarabine as consolidation therapy on an outpatient basis, for a total of 76 cycles. Admission was required in only 25% of cycles. Of note, the death rate was significantly higher in patients older than 50 years.25 In another retrospective study, the standard regimen of consolidation with high-dose cytarabine given on days 1, 3 and 5 was compared with a regimen in which cytarabine was given for three consecutive days (days 1, 2 and 3). Time to neutrophil and platelet recovery and duration of hospitalization were significantly shorter with the latter regimen.26 Strategies to minimize costs related to treatment and to improve patients’ quality of life are warranted.
The main limitations of the present study are its retrospective design and the small number of subjects. On the other hand, our study showed that in the setting of a Brazilian public hospital, high-dose cytarabine as consolidation therapy is feasible, with manageable toxicity. In addition, a careful selection of patients suitable to be discharged after chemotherapy may reduce costs associated with treatment. Finally, while the overall incidence of IFD after consolidation is low, the fatal case of candidemia in our cohort raises the question of antifungal prophylaxis during consolidation with high-dose cytarabine.
In conclusion, our data suggest that high-dose of cytarabine (18 g/m2) is a safe option after induction of AML.