Foetal anaemia is caused by a severe pregnancy complication, haemolytic disease of the foetus and newborn. Intrauterine transfusions (IUTs) are performed to treat foetal anaemia in alloimmunised pregnant women. If left untreated hydrops can develop thereby reducing the chance of survival. Survival rates have improved but the procedure is not without complications. Procedure-related complications can be associated with early gestational age, hence delaying IUT could improve outcomes. This review aims to determine the effectiveness and safety of IUTs by examining survival and mortality rates, procedure-related complications with associated foetal mortality and the influence of hydrops.
Study design and methodA systematic review was conducted by searching keywords in four scientific databases from January 2000 to April 2022. A meta-analysis was performed with the OpenMeta-Analyst software using an arcsine transformed proportion with the binary random-effects model and maximum likelihood method.
ResultsFifteen studies were identified as eligible and used in the meta-analysis. The forest plots all showed statistically significant outcomes with heterogeneity of data. Results indicated a greater foetal survival rate with IUT to treat anaemic foetuses, a low foetal mortality rate, and low risk of procedure-related complications associated with foetal loss but a higher risk of foetal mortality when hydrops is present.
ConclusionThe findings of this systematic review and meta-analysis provide evidence that IUT is a safe and effective treatment for foetal anaemia in the absence of hydrops when experienced personnel perform the procedure to minimise the risk of procedure-related complications.
Haemolytic disease of the foetus and newborn (HDFN) is a severe pregnancy complication, where maternal IgG antibodies from the mother cross the placenta and destroy foetal red blood cell (RBC) antigens. This is usually caused by an antibody to the Rh(D) antigen.1 As the maternal Rh(D)-negative RBCs are exposed to the Rh(D)-positive RBCs of the baby, Rh(D) alloimmunisation in the mother develops.2 This can lead to foetal anaemia and if untreated it can result in hydrops foetalis, hypovolemic shock and foetal death.3 Foetal anaemia is the main risk to foetal survival with severe Rh(D) alloimmunisation.4 Prophylactic anti-D immunoglobulin is standard treatment in the prevention of alloimmunisation thereby decreasing the rate of foetal anaemia, however it is not widespread in developing countries due to the lack of awareness and availability in remote areas.5,6 Also, once alloimmunisation occurs it cannot be administered during pregnancy.4 It is a modern practice to use maternal plasma in alloimmunised women to determine Rh(D) foetal antibodies. Foetal Rh(D) genotyping uses cell-free foetal DNA from maternal plasma to accurately determine foetal Rh(D) status, thus reducing unnecessary treatment with routine anti-D immunoglobulin and proceed to the appropriate screenings and treatment.7
High-risk pregnancies of foetal anaemia are identified via routine screening at 15 and 28 weeks of gestation to detect maternal antibodies.8 Life threatening foetal anaemia can be treated by intrauterine transfusions (IUTs).9 Treatment of foetal anaemia was developed by Liley in 1963 when an IUT intraperitoneal route was established to save pregnancies.10 Survival rates improved but the outcomes of hydropic foetuses were poor.13 The technique was improved by intravascular transfusions using fetoscopically cannulated vessels on the placental surface by Rodeck in 1981.11 This technique was then modified by Bang who introduced the ultrasound-guided foetal intravascular transfusion.12 This approach is beneficial to hydropic foetuses due to a reduction in the RBCs being absorbed from a peritoneal cavity affected by ascites.14 In 2000 the application of non-invasive middle cerebral artery peak systolic velocity and Doppler ultrasound measurements were accurate tools to predict the presence of foetal anaemia and to reduce the number of invasive procedures required.15,16 Foetal anaemia benefits from IUTs with survival rates of 94 % for non-hydropic foetuses and 74 % for hydropic foetuses.5,9 Mortality outcomes have improved due to the accuracy and reliability of predicting anaemia and the availability in utero treatment.
Procedure-related complications of intrauterine transfusionsIUTs have improved foetal survival but the procedure is not without its complications. Performing the IUT procedure requires a high level of skill and so reducing the number of transfusions is desirable to prevent the risk of procedure-related (PR) complications including the risk of infections transmitted via blood components.17,18 PR complications include membrane rupture, preterm delivery, chorioamnionitis, and foetal and neonatal death.8,19 The most serious complication during the procedure is foetal distress which can result in emergency preterm delivery or foetal death. Major complications from IUTs are associated with earlier stages of gestation (< 20 weeks) as foetal vessels are smaller, hence access to foetal circulation is difficult, and foetuses cannot tolerate the transfusion and die in utero due to acute haemodynamic changes.3,6 The risk of foetal death due to PR complications in gestations of less than 20 weeks is 8.5 % and of greater than 20 weeks it is 0.9 % per procedure.20 Foetuses with severe anaemia and hydrops have higher risks of foetal and neonatal death.19,21
Improving mortality outcomesPostponing IUT would be beneficial to reduce PR complications. As pregnancy progresses the foetal umbilical vein increases in diameter, making the procedure easier and safer.9 To delay the procedure, a high-dose of intravenous immunoglobulin (IVIG) can be administered. This was explored in a study where the onset of foetal anaemia was delayed by 8–17 weeks after the administration of high-dose IVIG, thus postponing the need for IUT.22 The mechanism of action of IVIG is unknown, but there are several proposed theories.23 IVIG theoretically may block the Fc receptors in the neonatal reticuloendothelial system and induce competition at the placenta, reducing Fc-mediated antibody transport. This prevents foetal haemolysis but does not treat existing foetal anaemia. Foetal mortality improved as IVIG appeared to reduce the mortality rate from 51 % to 20 %.17,20 Hence, theoretically, IVIG is beneficial but further studies on a larger scale are required to provide sufficient evidence.
Scope of reviewFoetal death is a devastating event that can occur when a mother is Rh(D) alloimmunised causing foetal anaemia. Ultrasound-guided IUT is a successful technique used to treat foetal anaemia thereby reducing the mortality rate. However, complications can arise and lead to preterm birth, membrane rupture and death of the foetus or neonate. These PR complications may be associated with gestational age. Hence, early diagnosis and identifying the optimal time to perform the procedure would be beneficial in order to start treatment before the development of hydrops thus enhancing the chances of survival.
Postponing the need and decreasing the frequency of IUTs could reduce the chance of PR complications; finding the best possible way to accomplish this remains unknown. There have been multiple studies that examined the use of high-dose IVIG as an alternative therapy however, its mechanism of action is not fully understood and this treatment does not eliminate foetal anaemia, it only helps to reduce foetal haemolysis. IVIG may be successful in delaying the onset of foetal anaemia and thus delay the need for IUT and reduce mortality.
The aim of this study was to determine whether IUT is a safe and effective treatment for foetal anaemia in Rh(D) alloimmunised pregnant women?
This study evaluated the mortality rate of foetuses affected by foetal anaemia due to severe Rh(D) alloimmunisation. The primary aim of this study was to investigate the survival and foetal mortality rates, to determine the effectiveness of IUTs and to examine the risk of PR complications associated to foetal loss so as to determine IUT safety and identify the influence of hydrops.
MethodsStudy designThe Preferred Reporting Item for Systematic Review and Meta-analysis (PRISMA) procedure was implemented in this systematic review to assist in gathering relevant articles to investigate the safety and effective management of IUTs in Rh(D) alloimmunised pregnant women.24
Search strategySearching for appropriate literature was conducted using PubMed, Scopus, Google Scholar and Semantic Scholar databases, with the timeline defined as from January 2000 to April 2022. Key search terms included “intrauterine transfusion” AND “alloimmunisation”, “intrauterine transfusion treatment”, “alloimmunisation pregnancy treatment”, “severe Rh(D) alloimmunisation”, “Rh(D) alloimmunised AND foetal anaemia” and “foetal anaemia AND treatment”. A manual search was performed within reference lists of selected articles with one additional study being identified. See Appendix 1 for full search strategy.
Eligibility criteriaTo determine the eligibility of the studies obtained in the search, articles were screened based on title and abstract with the application of inclusion/exclusion criteria. Articles that examined the management of IUT and evaluated the mortality rate of affected foetuses were considered eligible. Articles that reported PR complications with foetal outcomes and a comparison between the presence and absence of hydrops were also included. Exclusion criteria comprised of: 1) review articles and book chapters, 2) non-English publications, 3) inexistence of comparison group when investigating hydrops, 4) non-inclusion of foetal anaemia, 5) non-evaluation of mortality rates, 6) case reports, 7) abstract only articles.
Assessment of methodological qualityThe selected studies were assessed according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist for their methodological quality.25
Data extraction and managementData was extracted from each eligible study including the primary author, year of publication, study design, country, study period, sample size (both the total number of foetuses and the total number of transfusions performed) and the parameters measured for analysis. These parameters included survival rates, mortality rates, and PR complications and their associated foetal loss. Only data for survival and foetal mortality were used in the systematic review for articles that contained a comparison between the presence and absence of hydrops after the first IUT.
Statistical analysisTo conduct the meta-analysis, OpenMeta-Analyst software was downloaded via the Brown University website.26 A single-arm proportion analysis was used for overall survival, foetal mortality and PR complications with associated foetal loss parameters and a two-arm proportion analysis was used for the presence and absence of hydrops comparing the survival and foetal mortality rates. Each used the arcsine transformed proportion with the binary random-effects model and maximum likelihood method. The results for each parameter are presented as forest plots. The software calculated the overall p-value to ensure statistical significance with 95 % confidence intervals and I2 to assess heterogeneity and its corresponding p-value. An overall p-value < 0.05 was considered statistically significant.
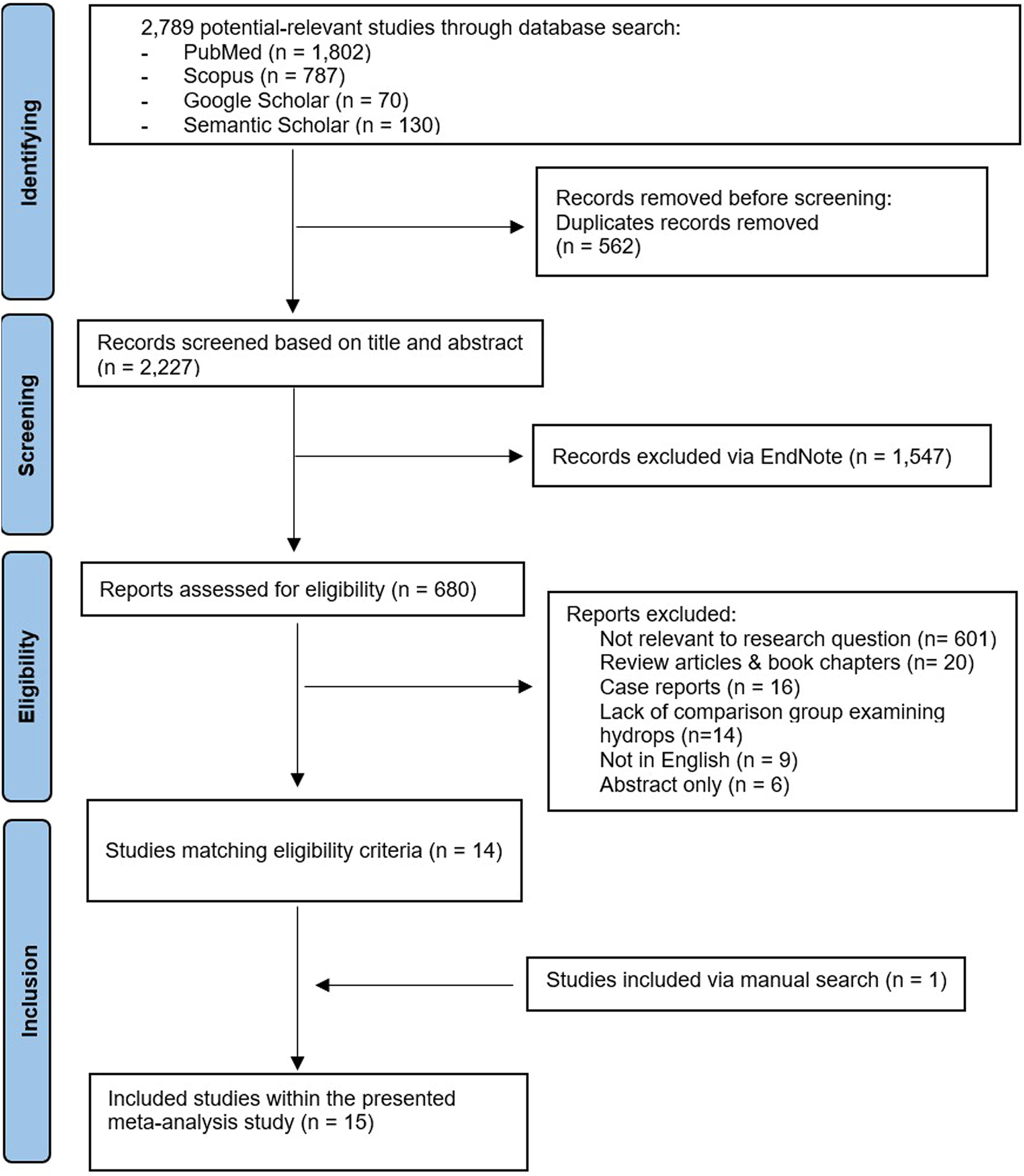
ResultsStudy selectionThe study selection based on inclusion/exclusion criteria is shown in Figure 1. The database search obtained 2789 potentially relevant studies. Duplicates were identified and removed leaving 2227 studies. These were screened based on title and abstract with 1547 studies being excluded. The remaining 680 studies were assessed for eligibility. Irrelevance to the research question, the major exclusion criteria, excluded another 601 studies. Review articles & book chapters, case reports, lack of a comparison group for hydrops, non-English publications and abstract only papers were also excluded. Thus, 14 studies matched the eligibility criteria, and one extra study was added after a manual search. All 15 studies were included in the meta-analysis.
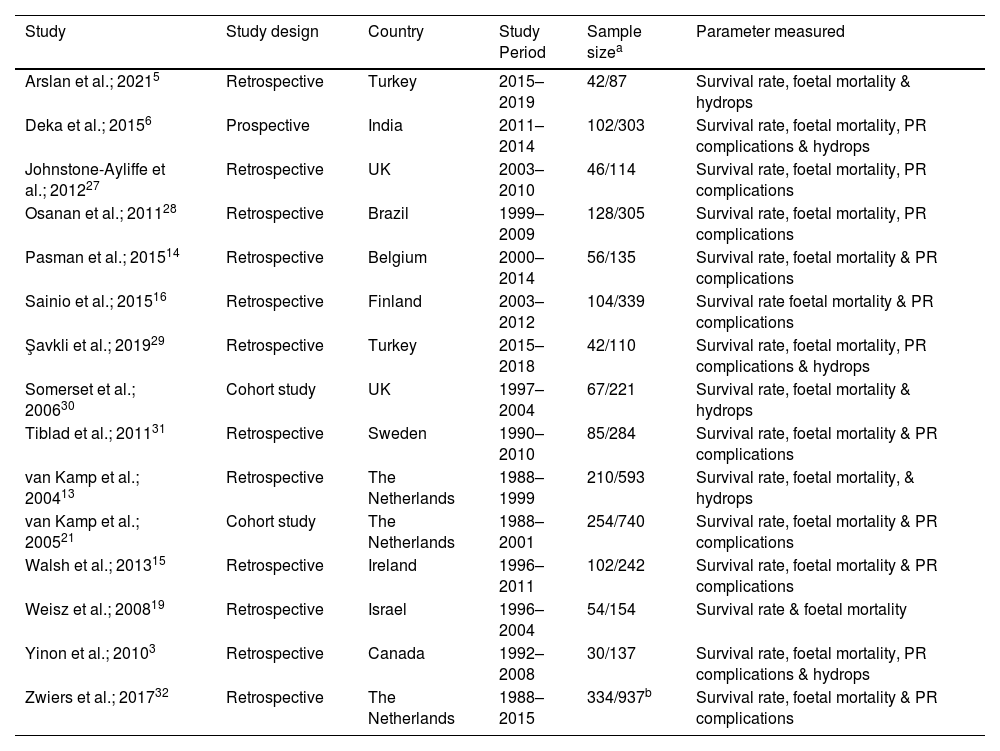
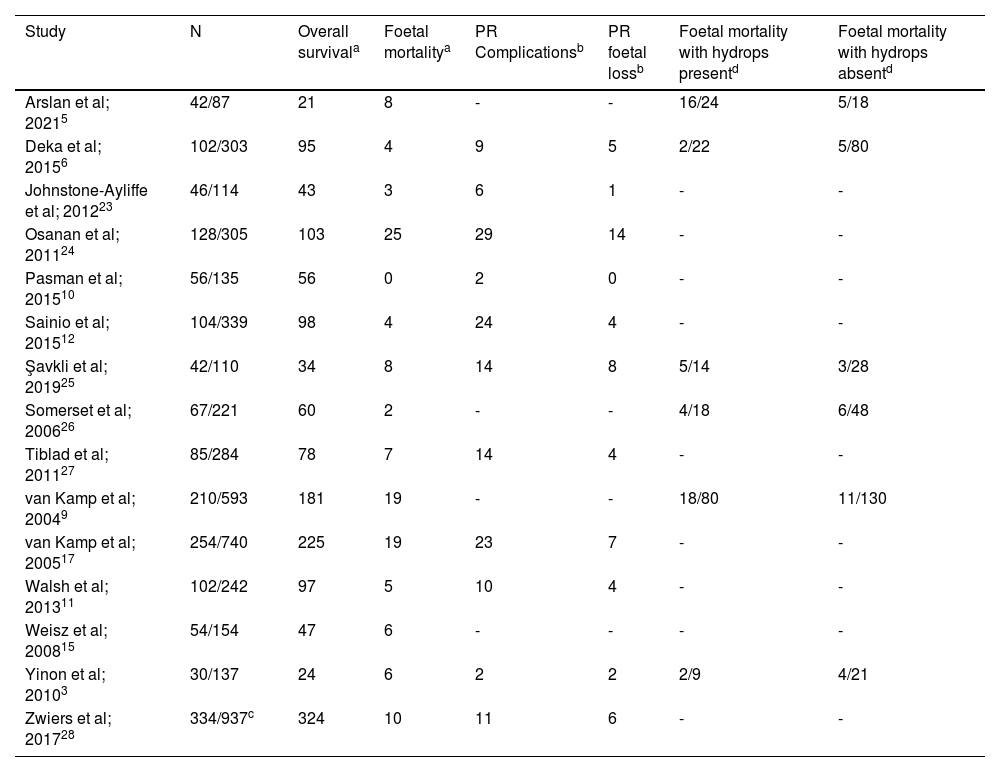
Study characteristicsThe characteristics of the 15 studies that evaluated the survival and mortality rates of anaemic foetuses are shown in Table 1. Of the included studies, 11 highlighted the risk of PR complications and foetal loss rates and six studies emphasised the influence of hydrops contributing to mortality. Most of these studies had a retrospective study design across a 1988 to 2019 time period spanning 11 different countries. The sample size included the total number of foetuses which ranged between 42 and 334, and the total number of transfusions which ranged between 87 and 937. The gestational age at first IUT for most studies was 25–28 weeks. However, one study conducted by Yinon et al. performed the first IUT at a mean gestational age of 20 weeks,3 and another study conducted by Tiblad et al. performed the first IUT at a mean gestational age of 31 weeks.31
Characteristics of eligible studies for the analysis of effective intrauterine transfusion treatment for foetal anaemia in Rh(D) alloimmunised pregnant women.
| Study | Study design | Country | Study Period | Sample sizea | Parameter measured |
|---|---|---|---|---|---|
| Arslan et al.; 20215 | Retrospective | Turkey | 2015–2019 | 42/87 | Survival rate, foetal mortality & hydrops |
| Deka et al.; 20156 | Prospective | India | 2011–2014 | 102/303 | Survival rate, foetal mortality, PR complications & hydrops |
| Johnstone-Ayliffe et al.; 201227 | Retrospective | UK | 2003–2010 | 46/114 | Survival rate, foetal mortality, PR complications |
| Osanan et al.; 201128 | Retrospective | Brazil | 1999–2009 | 128/305 | Survival rate, foetal mortality, PR complications |
| Pasman et al.; 201514 | Retrospective | Belgium | 2000–2014 | 56/135 | Survival rate, foetal mortality & PR complications |
| Sainio et al.; 201516 | Retrospective | Finland | 2003–2012 | 104/339 | Survival rate foetal mortality & PR complications |
| Şavkli et al.; 201929 | Retrospective | Turkey | 2015–2018 | 42/110 | Survival rate, foetal mortality, PR complications & hydrops |
| Somerset et al.; 200630 | Cohort study | UK | 1997–2004 | 67/221 | Survival rate, foetal mortality & hydrops |
| Tiblad et al.; 201131 | Retrospective | Sweden | 1990–2010 | 85/284 | Survival rate, foetal mortality & PR complications |
| van Kamp et al.; 200413 | Retrospective | The Netherlands | 1988–1999 | 210/593 | Survival rate, foetal mortality, & hydrops |
| van Kamp et al.; 200521 | Cohort study | The Netherlands | 1988–2001 | 254/740 | Survival rate, foetal mortality & PR complications |
| Walsh et al.; 201315 | Retrospective | Ireland | 1996–2011 | 102/242 | Survival rate, foetal mortality & PR complications |
| Weisz et al.; 200819 | Retrospective | Israel | 1996–2004 | 54/154 | Survival rate & foetal mortality |
| Yinon et al.; 20103 | Retrospective | Canada | 1992–2008 | 30/137 | Survival rate, foetal mortality, PR complications & hydrops |
| Zwiers et al.; 201732 | Retrospective | The Netherlands | 1988–2015 | 334/937b | Survival rate, foetal mortality & PR complications |
Note: PR: Procedure-related.
Study data used for the meta-analysis are shown in Table 2. The overall survival and foetal mortality rates were calculated per foetus and the PR complications and associated foetal loss were calculated per procedure. Data referring to mortality only relate to foetal mortality as neonatal morality was excluded. When investigating foetal mortality related to hydrops, data on survival and mortality of foetuses with or without hydrops were used to determine if it impacted survival. Data from one study only included results from the cohort from 2001 onwards.32
Data of eligible studies included in meta-analysis reporting proportion and risk of foetal mortality outcomes.
| Study | N | Overall survivala | Foetal mortalitya | PR Complicationsb | PR foetal lossb | Foetal mortality with hydrops presentd | Foetal mortality with hydrops absentd |
|---|---|---|---|---|---|---|---|
| Arslan et al; 20215 | 42/87 | 21 | 8 | - | - | 16/24 | 5/18 |
| Deka et al; 20156 | 102/303 | 95 | 4 | 9 | 5 | 2/22 | 5/80 |
| Johnstone-Ayliffe et al; 201223 | 46/114 | 43 | 3 | 6 | 1 | - | - |
| Osanan et al; 201124 | 128/305 | 103 | 25 | 29 | 14 | - | - |
| Pasman et al; 201510 | 56/135 | 56 | 0 | 2 | 0 | - | - |
| Sainio et al; 201512 | 104/339 | 98 | 4 | 24 | 4 | - | - |
| Şavkli et al; 201925 | 42/110 | 34 | 8 | 14 | 8 | 5/14 | 3/28 |
| Somerset et al; 200626 | 67/221 | 60 | 2 | - | - | 4/18 | 6/48 |
| Tiblad et al; 201127 | 85/284 | 78 | 7 | 14 | 4 | - | - |
| van Kamp et al; 20049 | 210/593 | 181 | 19 | - | - | 18/80 | 11/130 |
| van Kamp et al; 200517 | 254/740 | 225 | 19 | 23 | 7 | - | - |
| Walsh et al; 201311 | 102/242 | 97 | 5 | 10 | 4 | - | - |
| Weisz et al; 200815 | 54/154 | 47 | 6 | - | - | - | - |
| Yinon et al; 20103 | 30/137 | 24 | 6 | 2 | 2 | 2/9 | 4/21 |
| Zwiers et al; 201728 | 334/937c | 324 | 10 | 11 | 6 | - | - |
Note: N: number of foetuses / number of transfusions, PR: procedure-related
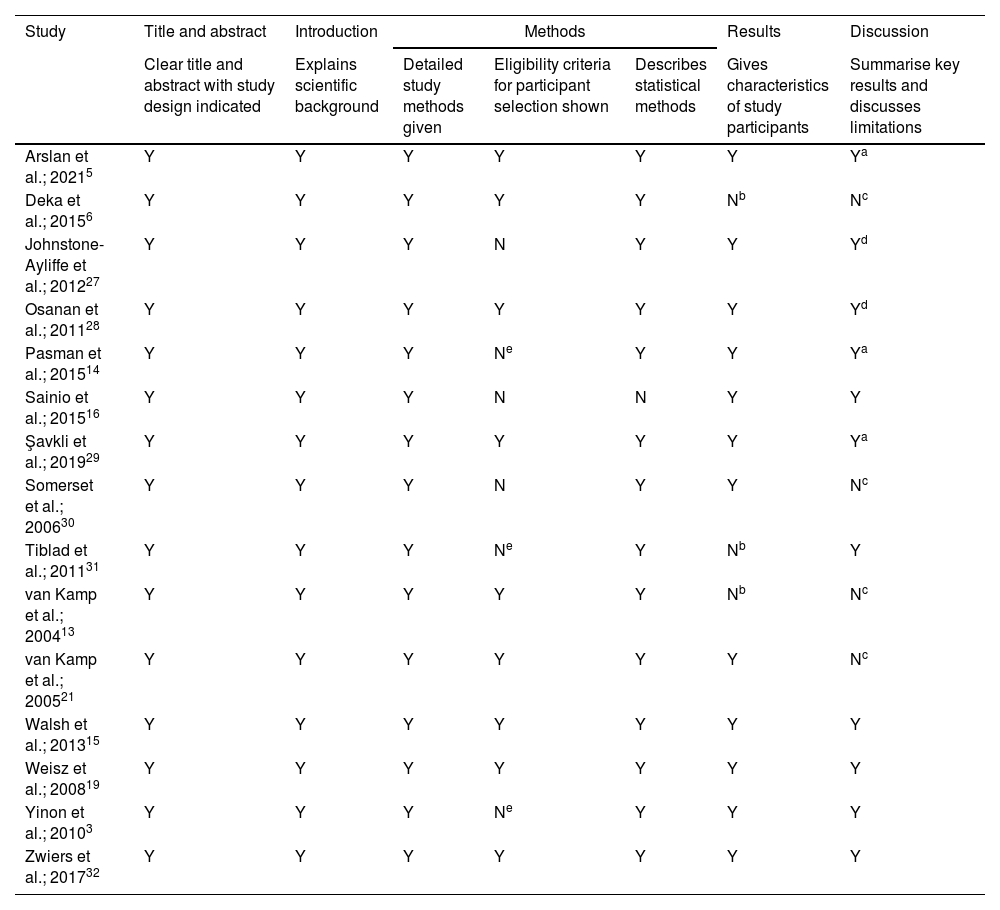
Studies included in the systematic review were analysed using the STROBE checklist to assess the methodological quality as shown in Table 3. All studies had a high quality providing sufficient detail for the title/abstract and introduction sections. Most studies had a high quality in the methods section, however two studies failed to outline eligibility criteria for participant selection and three studies only mentioned the inclusion criteria. A summary of participant characteristics was included in all studies however three had limited results. The majority of studies discussed limitations usually based on the retrospective study design or the sample size, however three studies failed to mention any limitations. Also, it should be noted that three studies failed to describe any efforts to address potential sources of bias within the methodology.3,19,30Table 3 shows that the studies were mostly of high methodological quality which reduces the risk of inappropriate study selection for the analysis.
Evaluation of methodological quality of included studies according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist.
| Study | Title and abstract | Introduction | Methods | Results | Discussion | ||
|---|---|---|---|---|---|---|---|
| Clear title and abstract with study design indicated | Explains scientific background | Detailed study methods given | Eligibility criteria for participant selection shown | Describes statistical methods | Gives characteristics of study participants | Summarise key results and discusses limitations | |
| Arslan et al.; 20215 | Y | Y | Y | Y | Y | Y | Ya |
| Deka et al.; 20156 | Y | Y | Y | Y | Y | Nb | Nc |
| Johnstone-Ayliffe et al.; 201227 | Y | Y | Y | N | Y | Y | Yd |
| Osanan et al.; 201128 | Y | Y | Y | Y | Y | Y | Yd |
| Pasman et al.; 201514 | Y | Y | Y | Ne | Y | Y | Ya |
| Sainio et al.; 201516 | Y | Y | Y | N | N | Y | Y |
| Şavkli et al.; 201929 | Y | Y | Y | Y | Y | Y | Ya |
| Somerset et al.; 200630 | Y | Y | Y | N | Y | Y | Nc |
| Tiblad et al.; 201131 | Y | Y | Y | Ne | Y | Nb | Y |
| van Kamp et al.; 200413 | Y | Y | Y | Y | Y | Nb | Nc |
| van Kamp et al.; 200521 | Y | Y | Y | Y | Y | Y | Nc |
| Walsh et al.; 201315 | Y | Y | Y | Y | Y | Y | Y |
| Weisz et al.; 200819 | Y | Y | Y | Y | Y | Y | Y |
| Yinon et al.; 20103 | Y | Y | Y | Ne | Y | Y | Y |
| Zwiers et al.; 201732 | Y | Y | Y | Y | Y | Y | Y |
Note: Y = criteria fulfilled; N = criteria not fulfilled.
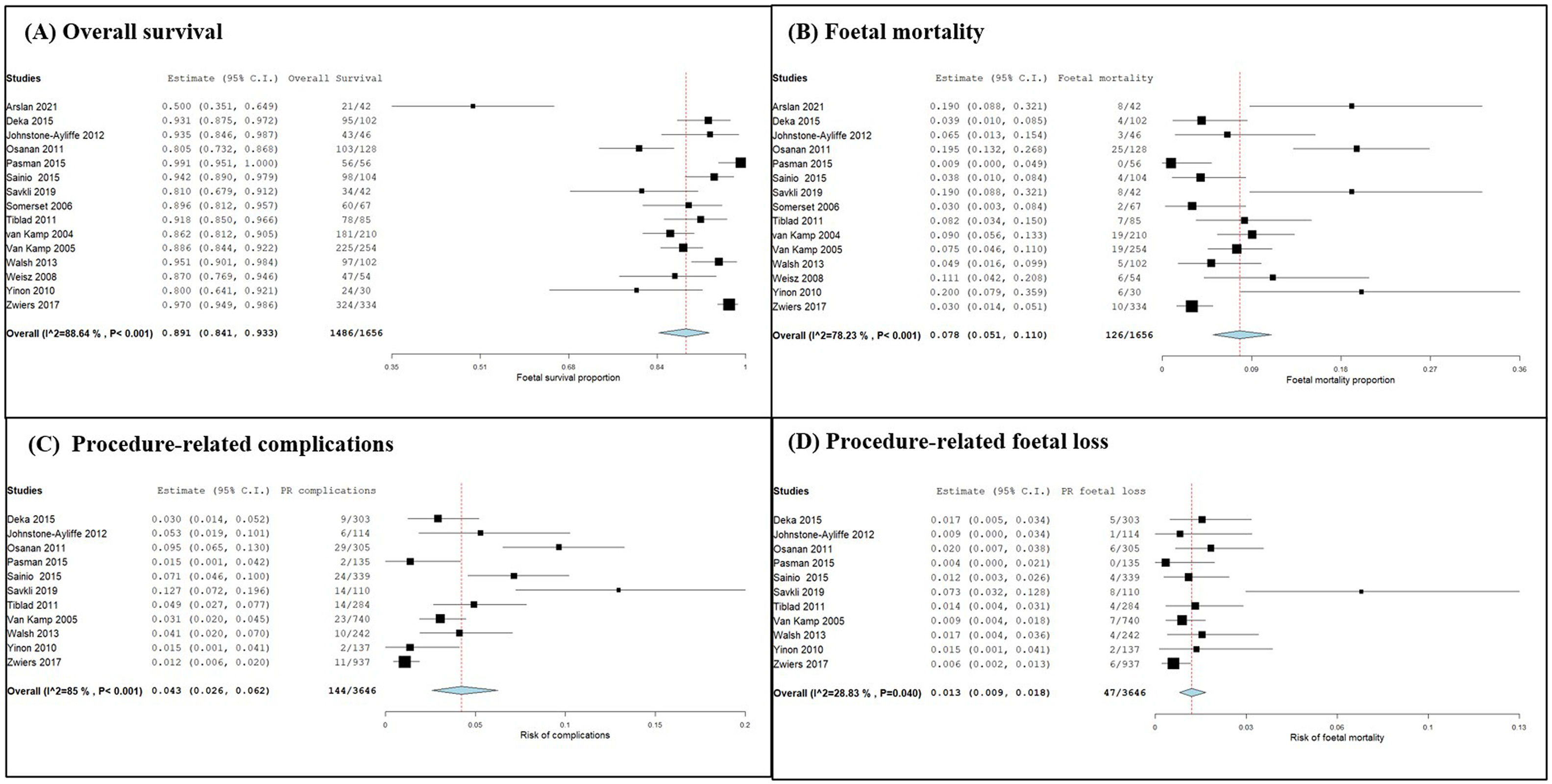
A meta-analysis and forest plots, constructed for overall survival, mortality rate, the risk of PR complications and the risk of foetal loss due to PR complications, are displayed in Figure 2, Parts A, B, C and D, respectively from the data presented in Table 2.
Forest plots of meta-analysis on survival and mortality rates, risk of complications and risk of foetal mortality. (A) Overall survival, (B) foetal mortality, (C) risk of procedure-related complications, and (D) risk of foetal loss due to PR complications were expressed after single-arm proportion data analysis over the total number of events with the arcsine transformed proportion, the binary random-effects model and maximum likelihood for each parameter evaluated with a 95 % confidence interval. Statistical significance was assessed by an overall p-value and heterogeneity was evaluated by the I2 value and associated p-value. Relative sample sizes were determined by weighting for each study.
The one-arm proportion analysis on overall survival was found to be statistically significant with an overall p-value < 0.001. The total survival was 89.79 % of foetuses. The results of the 15 studies were found to be highly heterogeneous (p-value < 0.001; I2 = 88.64 %) therefore, there is variability in the data. The results indicate that there is greater foetal survival when IUT is used to treat anaemic foetuses (95 % confidence interval [CI]: 0.841–0.933).
Similarly, the one-arm proportion analysis of foetal mortality was found to be statistically significant with an overall p-value < 0.001. The mortality rate was 7.61 % of foetuses. The results showed an I2 value of 78 % and a het p-value < 0.001 indicating high heterogeneity. The forest plot suggests a low foetal mortality rate with IUT treatment (95 % CI: 0.051–0.110).
Data on PR complications were used to determine the safety of IUT with a total PR complication rate of 3.95 %. Single-arm proportion analysis was conducted giving an overall p-value < 0.001 indicated the results were statistically significant, and an I2 value of 85 % and a het p-value of < 0.001 suggesting high heterogeneity. The results indicate a low risk, albeit not fully eliminated, of complications (95 % CI: 0.026–0.062).
Data on foetal loss due to PR complications (1.29 %) were also used to investigate the safety of IUTs. Single-arm proportion analysis was used to identify the risk of foetal mortality. An overall p-value < 0.001 indicates the results were statistically significant (95 % CI: 0.009–0.018). The results of the included studies were found to have low to moderate heterogeneity (p-value = 0.04; I2 = 28.8 %). The results suggest a low risk of foetal mortality due to PR complications.
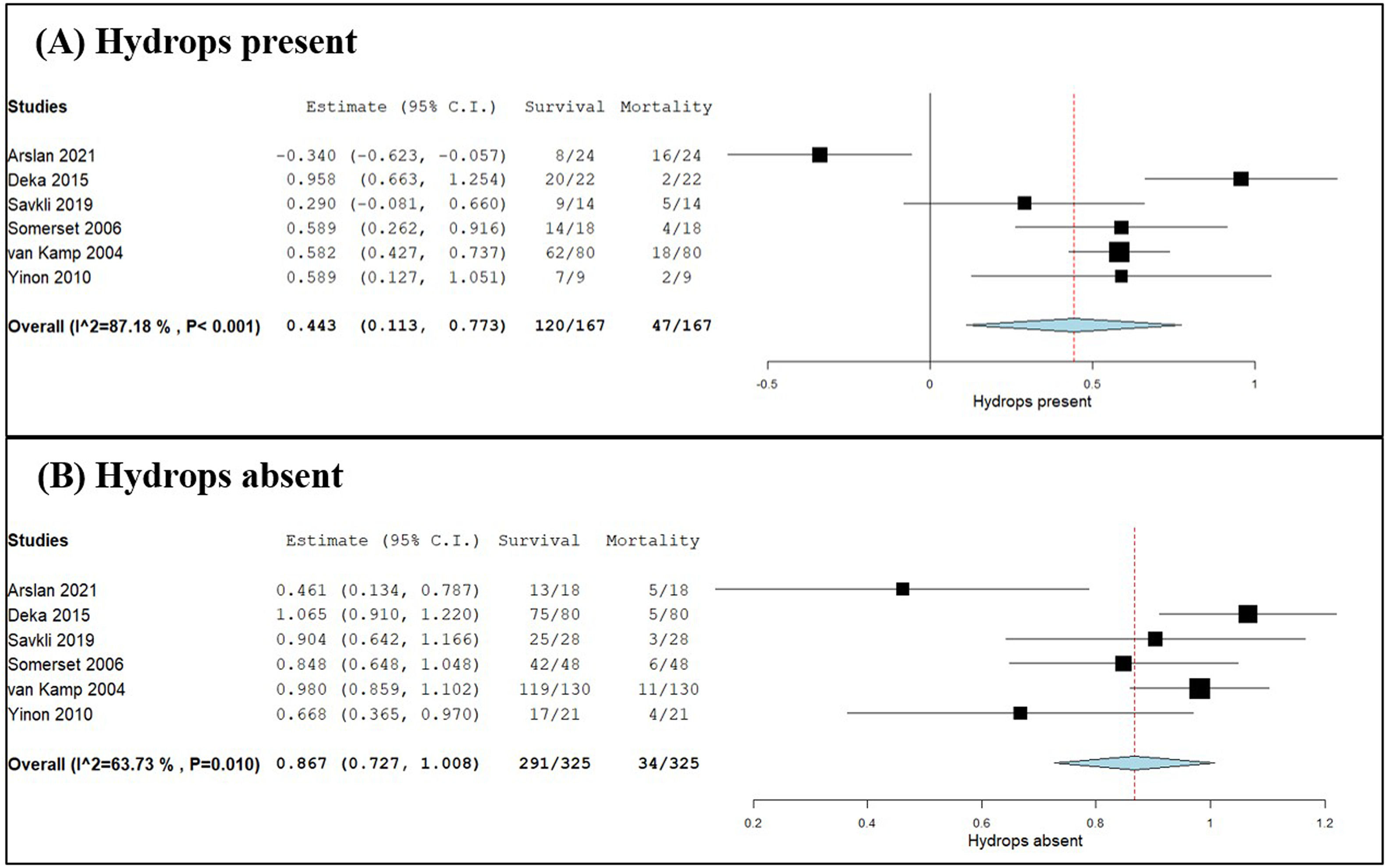
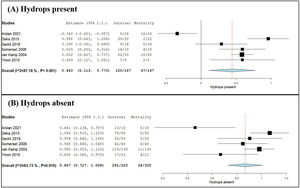
Meta-analysis of hydrops and risk of foetal mortalityThe influence of hydrops on foetal survival and mortality was examined by meta-analysis and forest plot as shown in Figure 3 (Part A: presence of hydrops; Part B: absence of hydrops). Two-arm proportion analysis was used for each parameter to compare the influence of hydrops using data on the survival rate and number of foetal mortalities compared to the total number of foetuses treated with IUT. Of the foetuses with hydrops, 71.86 % survived and 28.14 % died. Of those without hydrops, 89.54 % of the foetuses survived and 10.46 % died. The overall p-value of 0.009 calculated for foetuses with hydrops proved statistically significance with high heterogeneity (I2 = 87.18 %; het p-value < 0.001; 95 % CI: 0.113–0.773). The forest plot suggests a shift towards foetal mortality when hydrops is present. The results for foetuses without hydrops showed an overall p-value < 0.001 hence statistically significant (95 % CI: 0.070–0.135). However, the heterogeneity I2 value was 63.73 % with a het p-value of 0.010, which indicates moderate heterogeneity.
Forest plots of meta-analysis comparing survival & foetal mortality rates related to hydrops. (A) Hydrops present and (B) hydrops absent were expressed using two-arm proportion data analysis compared to the total number of events using the arcsine transformed proportion, the binary random-effects model and maximum likelihood for each parameter assessed with a 95 % confidence interval. Statistical significance was considered for an overall p-value with heterogeneity being evaluated by the I2 value and associated p-value. Relative sample sizes were determined by weighting for each study.
This systematic review and meta-analysis revealed with good power that IUT is an effective treatment for foetal anaemia. The overall survival rate was statistically significant and showed that 1486/1656 foetuses survived after IUT (95 % CI: 0.841–0.933: p-value < 0.001). The high heterogeneity is represented by I2 = 88.64 % and p-value < 0.001 categorising the variability of data across studies as heterogeneous rather than by chance. The results obtained can be explained by the number of studies included in the meta-analysis. The results provide confidence for reproducibility as highlighted by the narrow blue diamond on the forest plot. One of the studies showed a 50 % chance of survival which is a considerably different result to the other studies.5 This survival rate was the lowest recorded, but this was due to the large number of severely hydropic cases in the cohort. It was found that this study included severely anaemic foetuses at first IUT and the majority of participants had severe hydrops which probably impacted on the result. In addition, most of the mortality that occurred was neonatal and not foetal death. Overall, the results showed a greater rate of the anaemic foetuses survived after IUT treatment however, this is what the results suggest rather than the conclusion due to the high heterogeneity of the study.
Foetal mortality rates also supported IUT treatment. Results suggested a low risk of foetal mortality after IUT treatment. The overall foetal mortality rate of 126/1656 was found to be statistically significant (95 % CI: 0.051–0.110; p-value < 0.001) and had confidence for reproducibility. The results showed an I2 value of 78 % and a het p-value < 0.001 indicating high heterogeneity, hence variability present in the data with the results obtained being used as a suggestion rather than a firm conclusion. Most studies identified a similar low range of foetal death, but four studies showed higher weighting on mortality.3,5,27,29 These studies had more cases of hydrops and more procedures performed which could have led to an increase in PR complications hence, higher mortality. One of the studies had a mean gestational age of 20.4 weeks at first IUT with treatment being shown to be less effective.3
Safety of intrauterine transfusion and risk of procedure-related complicationsIUTs have proven to be a safe and beneficial procedure as results indicate a low risk of PR complications. A total PR complication rate of 144/3646 procedures was obtained by 11 studies and was found to be statistically significant with high heterogeneity (95 % CI: 0.026–0.062; p-value < 0.001; I2 = 85 %; het p-value < 0.001). Results suggest there is a low risk for PR complications, however there is an increased risk per procedure performed and some foetuses may require multiple transfusions. Hence, postponing the need for IUTs at early gestational ages would be beneficial to limit the frequency of transfusions. IVIG could be used as a non-invasive alternative to avoid early transfusions and delay the onset of foetal anaemia.20 Two studies showed a higher complication risk.28,29 These studies had a larger proportion of participants with hydrops which could have caused a shift in the results of the meta-analysis.
Data on foetal loss due to PR complications ensured that IUTs are safe procedures. The results indicate a low risk of foetal mortality due to PR complications. An overall PR foetal loss rate of 47/3646 procedures was obtained and was found to be statistically significant with low to moderate heterogeneity (95 % CI: 0.009–0.018; p-value < 0.001; I2 = 28.83 %; het p-value = 0.04). This suggests that the studies reported comparable results, with confidence of reproducibility; there is a low risk of foetal mortality due to PR complications. One of the studies showed a much higher risk of foetal loss.29 It was found that 33 % of foetuses had hydrops, some of the foetuses were treated at an early gestational age of 22 weeks and all foetuses required more than one IUT. There can be difficulty to access small vessels for transfusions at early gestational ages, hence a higher foetal mortality rate. Therefore, treatment is not as effective in early gestation. Delaying IUT to a later gestational age with IVIG would be beneficial as the vessel diameter would have increased, making the procedure easier and safer; this could also reduce the number of IUTs required per foetus.20 The mechanism of action of IVIG is unknown, but theoretically it stimulates competition at the placenta by reduces Fc-mediated antibody transport, preventing foetal haemolysis. However, this treatment cannot treat existing foetal anaemia on its own and so IUTs are still required.23
Influence of hydropsThe presence of hydrops can influence foetal mortality. Hydrops occurs if the baby's developing organs struggle to overcome anaemia, leading to heart failure and death. The condition has a higher risk of intrauterine foetal death and is harder to treat.33 Data from six studies were used to compare the survival and mortality data in respect to the total number of foetuses being treated by IUT; hydrops was present in 120/167 foetuses that survived and in 47/167 foetal mortalities. The results were statistically significant with high heterogeneity (95 % CI: 0.113–0.773; p-value = 0.009; I2 = 87.18 %; het p-value < 0.001) indicating variation in the data. The results suggest a shift towards foetal mortality when hydrops is present. Hydrops was absent in 291/325 foetuses that survived and 34/325 foetal mortalities. Even though there is a higher survival rate for foetuses without hydrops, it does not exclude the risk of mortality. Multiple IUTs had been performed for the 34 foetuses that died in utero, hence an increased risk of PR complications exists. The results were statistically significant with moderate heterogeneity (95 % CI: 0.070–0.135; p-value < 0.001; I2 = 63.73 %; het p-value = 0.010), indicating some variation between studies. The results suggest that there is a better chance of survival when hydrops is absent. Therefore, early detection of anaemia and the prevention of the development of hydrops are important to increase the chance of survival.
Limitations of reviewThe major limitation of this review was the lack of prospective studies. The included studies were mainly retrospective which can introduce bias. Some of the studies failed to address potential sources of bias and discuss any limitations that might have existed. Some studies only had one specialist conducting the study and gathering results from patients, however the majority of selected studies eliminated this element of bias by having multiple specialists oversee the study. Some studies also relied on previous medical records, hence assumed that the documentation had been filled out correctly. Finally, the hydrops data in the meta-analysis only included six studies, therefore there were less variations in the data.
ConclusionThis systematic review and meta-analysis identified with good power that IUT is an effective and safe treatment for foetal anaemia in Rh(D) alloimmunised pregnant women. It can be concluded that there is a greater survival rate after IUTs, low risks of PR complications and associated foetal loss, however there is a higher risk of foetal mortality when hydrops is present. Increasing gestational age and reducing the frequency of IUTs would improve survival. The results in this review can be used as evidence that IUTs are safe but require a high level of skill to perform, therefore it is important that specialists have the correct training and knowledge. The data in this review can also be used as evidence for health professionals to ensure safe and effective practices of IUTs to minimise the risk of PR complications and foetal mortality. Further research on other treatment options for foetal anaemia in early pregnancy is still required.
FundingThe authors received no financial support for the research
The search terms used to obtain appropriate literature for this systematic review and meta-analysis to determine the effective management of foetal anaemia in Rh(D) alloimmunised pregnant women using intrauterine transfusions are presented below. The following scientific databases were used with a timeline from January 2000 to April 2022. All searches were conducted in April 2022.
PubMed, Scopus, Google Scholar & Semantic Scholar
Search terms include:
“Intrauterine transfusion”, “intrauterine transfusion AND alloimmunisation”, “alloimmunisation AND pregnancy treatment”, “severe Rh alloimmunisation AND intrauterine transfusion”, “intrauterine transfusion OR pregnancy treatment”, “foetal anaemia AND pregnancy treatment”, “foetal anaemia AND intrauterine transfusion”, “intrauterine transfusion foetal survival”, “intrauterine transfusion AND mortality”, “Rh(D) alloimmunised AND foetal anaemia”, “intrauterine transfusion OR intravenous immunoglobulin”, “procedure related complications AND intrauterine transfusion”, “hydrops AND Rh(D) alloimmunisation”, “hydrops AND intrauterine transfusion”.
All articles were saved in EndNote. This software was used to identify and remove duplicates and provided assistance in the inclusion/exclusion criteria. Records were screened based on title and abstract and then assessed for eligibility. Reports were excluded if they were 1) not relevant to the research question, 2) review articles & book chapters, 3) case reports, 4) lacking a comparison group for hydrops, 5) non-English publications and 6) abstract only papers.
A manual search was also performed within reference lists of the selected articles with one additional study being added.