B-lymphoblastic lymphoma (B-LBL) is a clonal hematological malignancy of precursor lymphoid cells committed to B-cell lineage. B-LBL constitutes 2% of the lymphomas diagnosed in western world.1 The current WHO classification defines B-ALL/LBL as a disease spectrum, with the presentation in B-LBL being confined to extramedullary sites, and <25% blasts in bone marrow with absence of peripheral blood involvement.2 The commonest sites of involvement reported in B-LBL are lymph nodes, bone and skin (in 75% of the cases), with the remainder occurring in miscellaneous sites such as head & neck (parotid gland, Waldeyer ring), retroperitoneum, breast, ovary, brain, and soft tissues.3,4 Though incidental findings of pancreatic involvement have been described in few pediatric and adult B-ALL patients,5-11 there is no previous report of B-LBL with concurrent involvement of pancreas and kidneys. Herein we describe a very rare case of B-LBL with concurrent pancreatic and bilateral renal involvement, mimicking acute pancreatitis.
CaseA previously healthy young adult aged 20 years, presented to surgical outpatient department (OPD) with a short history spanning over 20 days, of acute onset epigastric pain radiating to the back, and associated with multiple episodes of non-bilious and non-projectile vomiting. There was no history of fever, bone pain, hematemesis, melena, jaundice, diarrhea, constipation, headache, or weight loss. His past medical and surgical history and family history were unremarkable. On examination, patient was conscious, oriented and of average build, with stable vitals, his performance status was ECOG 1.There was no pallor, icterus, sternal tenderness, peripheral lymphadenopathy, hepatosplenomegaly, features of raised intracranial pressure, or any focal neurological sign in the patient. An ill-defined, tender swelling was palpable in epigastrium.
During routine hematological and biochemical work-up complete blood counts showed hemoglobin of 12 g/dl, total leucocyte count of 5300/µl, platelet count of 213,000 /µl, normal differential leukocyte count, and no blasts were seen on peripheral blood smear examination. Serum amylase and lipase levels were raised (228 U/L and 616 U/L respectively), liver and renal function tests were within normal limits, and serum LDH was elevated (1444 U/L).
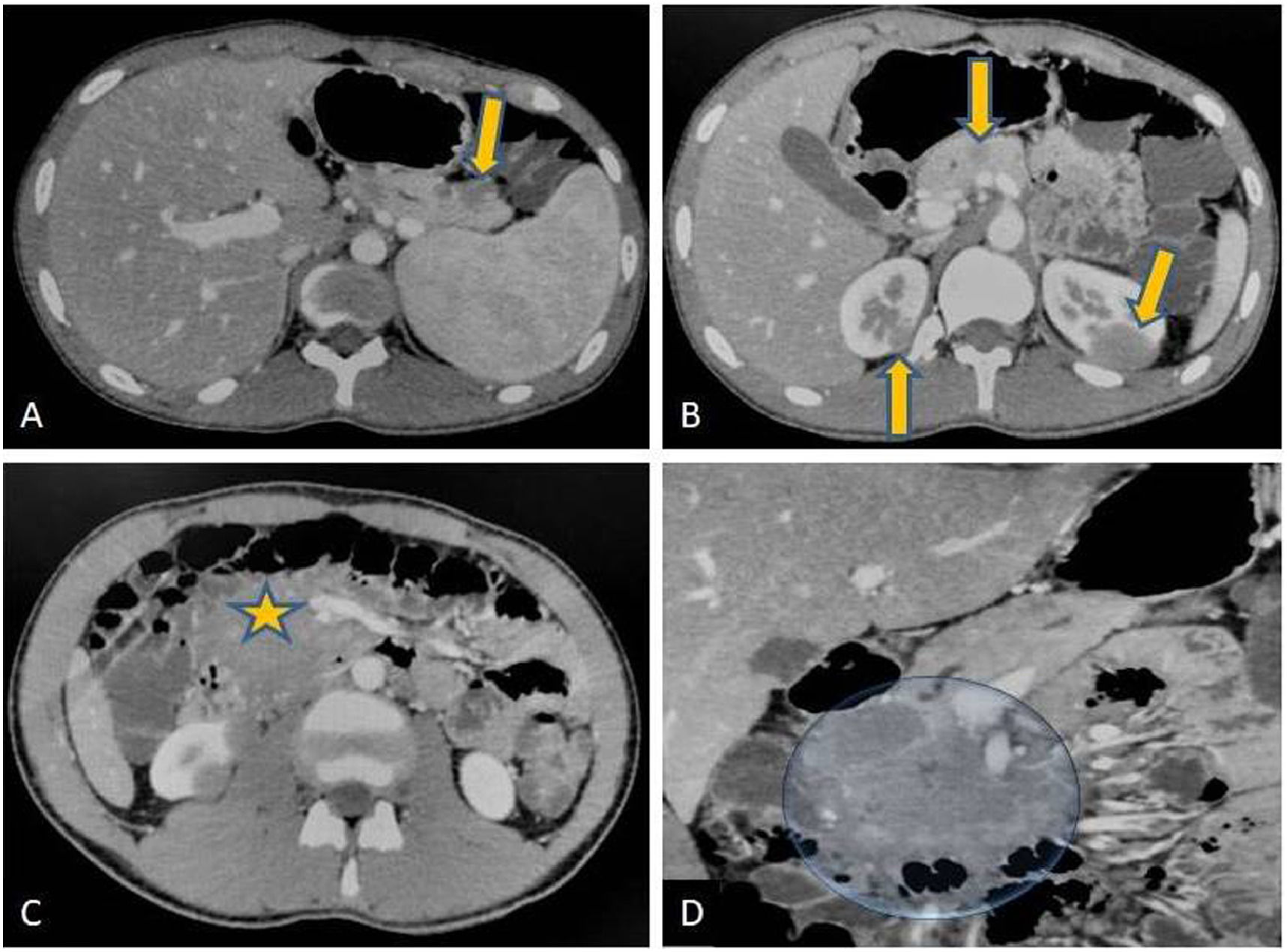
Contrast-enhanced CT scan (CECT) of abdomen revealed a well-defined, hypodense and hypo-enhancing mass lesion of size 5.4 × 6.8 × 6.6 cm in the pancreatic head without any calcification, few non-enhancing necrotic areas within the uncinate process, and dilated pancreatic duct (Figure 1). Smaller similar lesions were also noted in the body and tail of pancreas. An enhancing peri-pancreatic lymph node measuring 2.2 cm was also seen. Bilateral kidneys showed multiple hypo-enhancing lesions, largest measuring 2.8 × 2.5cm at lower pole of right and 2.7 × 2.9 cm at upper pole of left kidney. Liver, spleen, adrenal glands, bowel loops, urinary bladder and vertebrae did not show any focal lesion. Based on the discussed radiological findings a possibility of primary pancreatic lymphoma with bilateral renal deposits was suggested. Following this an ultrasound-guided biopsy from pancreatic mass was performed.
Axial (A, B, C) and coronal (D) contrast enhanced CT scans of abdomen. Well defined hypodense lesions seen in the tail and neck region of pancreas (arrows in ‘A’ and ‘B’) without any obvious evidence of mass effect. Similar lesions are noted at upper pole regions of both the kidneys (arrow in ‘C’).There is a large hypodense mass lesion in upper retroperitoneum with loss of fat planes with head of pancreas (star in ‘C ‘and marked in ‘D’). Patient also had hepatosplenomegaly. Radiological differential of lymphoma likely Non Hodgkin type was suggested.
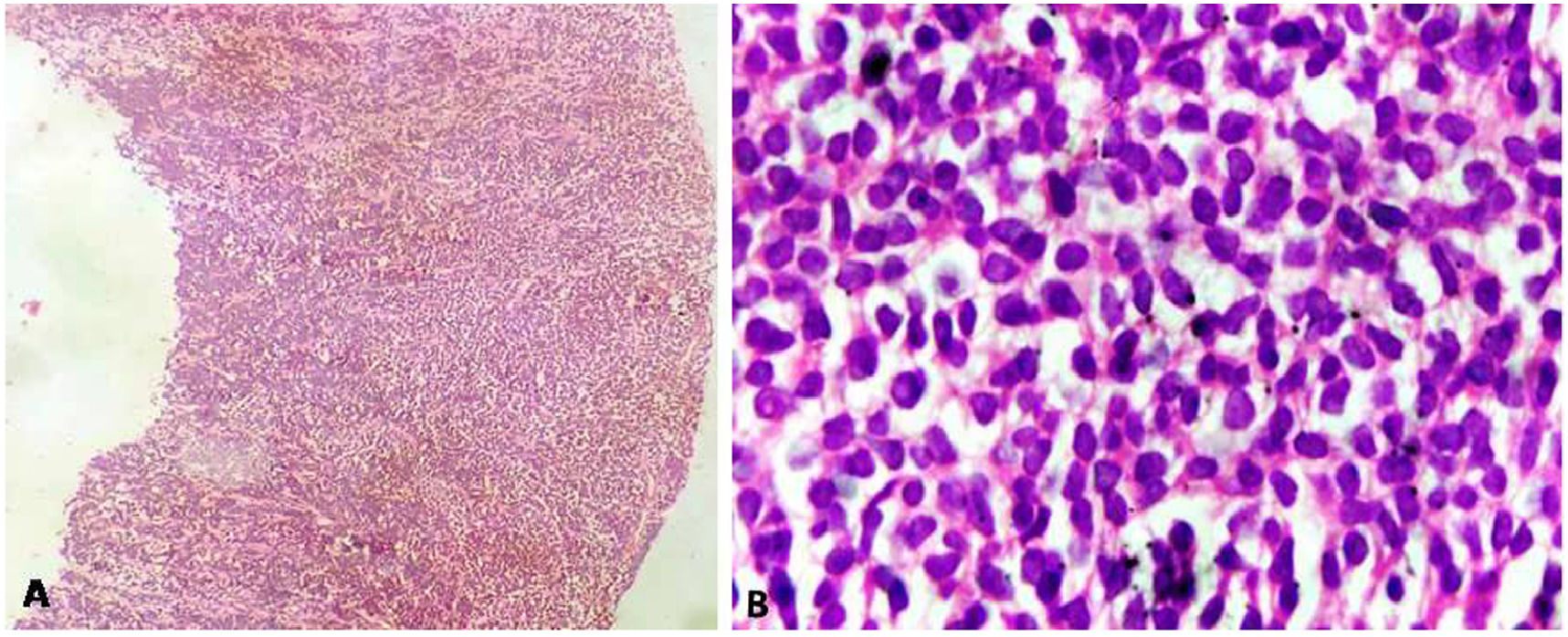
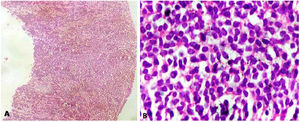
On haematoxylin and eosin-stained (H&E) section, a tumor comprising of small to medium sized monomorphic tumor cells arranged in diffuse sheets and focally in perivascular pattern was seen (Figure 2). The tumor cells had round to convoluted nuclei, dense chromatin, inconspicuous nucleoli and scant basophilic cytoplasm. Brisk mitotic activity was noted. A provisional morphological diagnosis of small round cell tumor was rendered.
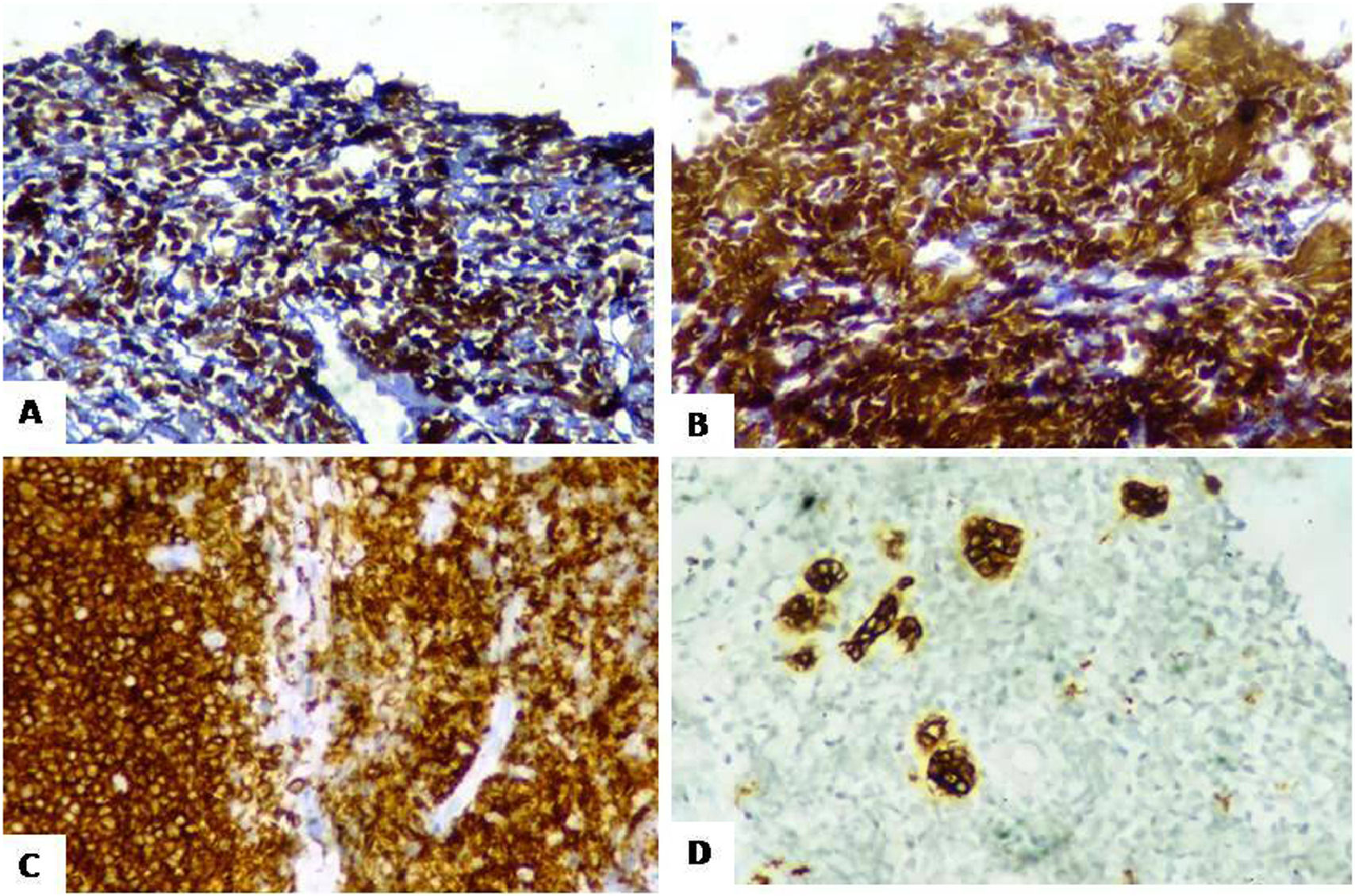
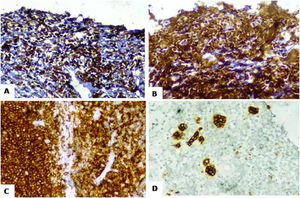
On immunohistochemistry (IHC), tumor cells were positive for Tdt (strong diffuse nuclear expression) and focally expressed CD34 and CD20 (Figure 3). Strong and diffuse expression was also noted for B-cell markers CD79a (membranous), CD19 (membranous) and Pax-5 (nuclear). The tumor cells were negative for CD45, CD3, HLA-DR, MPO, synaptophysin, chromogranin, pan-CK, WT1, desmin and myogenin. Histopathological examination and IHC were consistent with diagnosis of precursor B-lymphoblastic lymphoma.
Following this a bone marrow aspiration and biopsy of the patient was planned to rule out any evidence of marrow infiltration by lymphoma cells which revealed normo cellular marrow with normal trilineage hematopoiesis, without any infiltration.
Bone marrow flow cytometry was also done which did not reveal any leukemic clone, while cytogenetics showed a 46, XY karyotype. The patient however was lost to follow up. He returned 5 months after the initial visit with disease progression. CECT thorax and abdomen performed during the second visit showed pancreatic, bilateral renal and scrotal mass lesions, right renomegaly and vascular encasements. Bulky right shoulder, chest wall and left pectoral muscles with cortical irregularity of right scapula and left clavicle were also notable. He presented with non-tender, 8 × 5 cm bilateral testicular swellings, USG of which was suggestive of lymphoma infiltration. He also had multiple tender annular and erythematous to hyperpigmented nodulo-plaques measuring 5-10 cm, on skin over left flank, right anterior chest and right shoulder regions.
Biopsies were performed from the skin lesions over left flank and chest. Histopathological evaluation revealed infiltration by undifferentiated small round blue cell in the papillary as well as reticular dermis. Epidermotropism was not seen. IHC was consistent with diagnosis of B-LBL/leukemia cutis. A repeat bone marrow aspiration and biopsy revealed normocellular marrow for age with normal trilineage hematopoiesis. There was no evidence of marrow infiltration by myeloproliferative/lymphoproliferative disease. Bone marrow flow cytometry and cytogenetics were again unremarkable.
Cerebrospinal fluid (CSF) study revealed increased CSF cell count (680/µl) with presence of 90% lymphoblasts, suggestive of leptomeningeal involvement. The patient achieved complete remission with R-Hyper-CVAD (rituximab, plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, & dexamethasone, alternating with high-dose methotrexate & cytarabine) regimen chemotherapy, and triple intrathecal chemotherapy for leptomeningeal disease. At present the patient is on maintenance chemotherapy as per the protocol, and is doing well on follow up.
DiscussionThe diagnosis of B-LBL and its distinction from ALL is based on demonstrating focal involvement (<25% blasts) or absence of bone marrow and peripheral blood involvement, and presentation in the form of bulky tumor masses in solid organs.12
In context of B-LBL, clinical and histopathological scenario like present case has been infrequently described as majority of extramedullary lymphomas are of mature B cell lymphomas. On literature search and excluding cases in which there was bone marrow involvement by > 25% blasts, till date 105 documented cases of B-LBL were found in the form of single case reports and case series. In the two largest case series on precursor B lymphoblastic lymphomas by Lin et al.4 and Maitra et al.3 comprising of 25 cases and nine cases respectively, only one case with isolated pancreatic involvement was described by Maitra et al.
After an extensive search of the archives, it was found that there is no reported case of B-LBL with concurrent involvement of pancreas and kidneys. Hence, the present case is an extremely rare presentation of B-LBL.
The most common site of involvement by B-LBL in previous studies were skin (75% patients), lymph nodes, mediastinum, bone and miscellaneous sites including parotid gland, tonsils, breast, ovary, brain, retroperitoneum and soft tissues in a minority of cases.3
Without the aid of an extensive immunohistochemical analysis, morphologically it may be impossible to arrive to a confirmed diagnosis of B-LBL and to differentiate it from other retroperitoneal mass lesions which may present in pancreas of an adult male (Table 1).
IHC panel for a retroperitoneal small round cell tumour in adults.
DSRCT: Desmoplastic small round cell tumour; SS: Synovial sarcoma; RMS: Rhabdomyosarcoma; EWS: Ewing sarcoma; PNET: Peripheral neuroectodermal tumour.
Occurrence, of B-LBL in the pancreas and kidneys which are an extremely unusual site, clinically mimicking acute pancreatitis and disease advancement to gradually and sequentially involve multiple extramedullary sites is peculiar to the present case. This case also highlights that though rare but clinical suspicion of lymphomatous infiltration should be raised in an unexplained pancreatitis especially when showing concurrent lymph node and other visceral involvement on radiology.
The management of B-LBL comprises of aggressive multi-agent chemotherapy regimens used to treat acute lymphoblastic leukemia. Lin et al in their study found that B-LBL has a more favorable complete remission rate and remission duration compared to adult B-ALL treated with similar regimens.4 Few therapeutical trials have shown hyper CVAD regimen to be equally effective in B lymphoblastic lymphoma as in a ALL. Certain modification can be made in this regimen in slow responders and cases of CNS relapse.13 Patient in the present case had a favorable outcome with rituximab plus Hyper-CVAD chemotherapy regimen.
ConclusionThis case emphasizes that B-LBL, although a rare malignancy, must be included in the differential diagnosis of uncommon visceral mass lesions, as early diagnosis and aggressive treatment is usually associated with favorable outcomes.