To comparatively analyze maternal and fetal factors and quality markers of blood samples in a public umbilical cord blood bank.
MethodThis is a cross-sectional descriptive study that revisited 458 records of donations from September 2009 to March 2013 at the Hemocentro de Santa Catarina. The means of markers were used to define cutoff points for the quality of cord blood.
ResultsMost donations came from women with ages between 18 and 29 years (62.8%), gestational age≥40 weeks (55.2%), vaginal delivery (51.3%), primiparous (41.4%), and with male newborns (54.4%) weighing between 3000 and 3499g (41.8%). The volume of the donations ranged from 71.6 to 275.2mL, the total nucleated cell count ranged from 4.77×108 to 31.0×108 cells and CD34+ cells ranged from 0.05 to 1.23%. There were statistically significant differences in the volume with respect to gestation age>38 weeks (p-value=0.001), cesarean section (p-value<0.001) and birth weight>3500g (p-value<0.001). The total nucleated cell count was positively affected by cesarean section (p-value=0.022) and birth weight>3500g (p-value<0.001). There was no statistically significant difference between the variables and the percentage of CD34+ cells.
ConclusionsDelivery route and birth weight influence the volume of cord blood and the total nucleated cell count. Gestational age influences only the volume of cord blood.
Umbilical cord blood, which previously used to be discarded, can be collected shortly after birth and has great capacity to reconstitute the hematopoietic system.1 Several studies have shown the simplicity of umbilical cord blood collection, in addition to the lack of risk for both mother and newborn, low risk of graft-versus-host disease and low risk of transmitting infectious-contagious diseases.2,3 Furthermore, it is a useful alternative of hematopoietic stem cells for transplantation to treat diseases of the blood, immune system and for genetic disorders.3,4
One of the limitations of this type of transplant is the volume and contents of the blood collected from the umbilical cord which is an obstacle to hematopoietic stem cell grafting. The main parameters used in umbilical cord blood banks include the total nucleated cell (TNC) count, percentage of CD34+ cells, and the volume of blood.5
Transplantations using umbilical cord blood are still in the research phase and variables that might improve the quality of blood are currently the focus of research, inasmuch as knowing these factors may result in lower costs and less waste of time in the evaluation, processing and storage of material.6,7
Recent studies report that some variables affect the quality of the umbilical cord blood, especially those related to maternal and fetal features such as placental weight, birth weight, gestational age (GA), route of delivery, gender of the newborn, among other things; thus research is being developed in this area to attempt to improve cell levels, which is essential to increase grafting success rates.
ObjectivesThe aims of this study were to determine maternal and fetal characteristics of umbilical cord blood donors, to evaluate quality markers of umbilical cord blood, as well as to determine associations between maternal and fetal characteristics and these quality markers.
MethodsThis is an observational, cross-sectional, epidemiological study that revisited 458 charts of patients who, between September 2009 and March 2013, donated umbilical cord blood at the Blood Umbilical Cord and Placental Bank (BSCUP) of the Hemocentro de Santa Catarina (HEMOSC), located in the city of São José, Santa Catarina. Nine medical records of patients who performed allogeneic donations were excluded. Data collection began after the study was approved by the Research Ethics Committee of HEMOSC, and data were collected from computerized registers in the Cryobiology Sector of HEMOSC, located in Florianópolis, Santa Catarina.
Donation candidates were submitted to the standard survey of HEMOSC before collection in order to assess personal history, family history, and laboratory results with standard questions used for all kinds of blood donation. All patients who agreed to donate signed an informed consent form before the collection.
A data collection instrument was developed exclusively for this study which investigated the maternal age, gestational age, route of delivery, number of previous pregnancies, gender and newborn weight. Moreover, the preprocessing (blood volume) and post-processing data (TNC and CD34+ counts) of the umbilical cord blood were studied. The means of markers were used to define cutoff points for the quality of cord blood.
Blood collection was performed extra-utero and carried out by a trained professional in a separate room. As soon as placental delivery occurs, the placenta is taken to an area reserved for collection where the umbilical cord is cleaned with an antiseptic solution and the blood is drained by gravity through the most distal puncture site. The blood is stored in a standard blood bag containing citrate-phosphate-dextrose anticoagulant.
Once collected, these samples are labeled and sent to blood banks, where they undergo safety testing, HLA typing and cryopreservation. Typically, collections are kept for a time not exceeding 24–28h at 22±2°C before processing, and then cryopreserved in liquid nitrogen, under controlled freezing rates and stored long term in accordance with international criteria at a temperature lower than −150°C.1,8,9 The umbilical cord blood was processed within a maximum of 48h after being collected as is determined by the National Health Surveillance Agency (ANVISA).2
Collected data were stored in the Microsoft Excel computer program and later exported to the Statistical Package for the Social Sciences (Version 16.0) for analysis.
Qualitative variables are described as absolute and relative frequencies, while quantitative variables are described as means±standard deviations.
The Chi-squared test (χ2) or Fisher's exact test was used to test the homogeneity of proportions. Prevalence ratios (PR) and 95% confidence intervals (95% CI) were calculated. The level of significance was set for a p-value <0.05.
ResultsMost donations came from women with ages from 18 to 29 years (62.8%) and from 30 to 34 years (24.4%), gestational age≥40 weeks (55.2%), vaginal delivery (51.3%), primiparous (41.4%), and with male newborns (54.4%) weighing between 3000 and 3499g (41.8%).
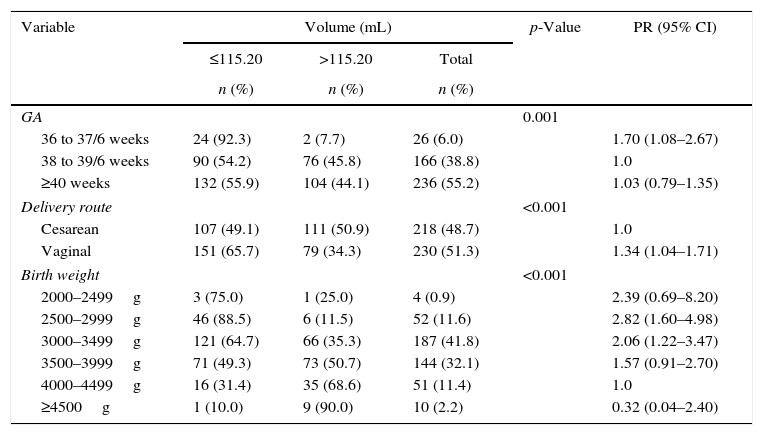
Comparisons between maternal and fetal characteristics and cord blood volume are shown in Table 1.
Maternal and fetal characteristics and cord blood volume.
| Variable | Volume (mL) | p-Value | PR (95% CI) | ||
|---|---|---|---|---|---|
| ≤115.20 | >115.20 | Total | |||
| n (%) | n (%) | n (%) | |||
| GA | 0.001 | ||||
| 36 to 37/6 weeks | 24 (92.3) | 2 (7.7) | 26 (6.0) | 1.70 (1.08–2.67) | |
| 38 to 39/6 weeks | 90 (54.2) | 76 (45.8) | 166 (38.8) | 1.0 | |
| ≥40 weeks | 132 (55.9) | 104 (44.1) | 236 (55.2) | 1.03 (0.79–1.35) | |
| Delivery route | <0.001 | ||||
| Cesarean | 107 (49.1) | 111 (50.9) | 218 (48.7) | 1.0 | |
| Vaginal | 151 (65.7) | 79 (34.3) | 230 (51.3) | 1.34 (1.04–1.71) | |
| Birth weight | <0.001 | ||||
| 2000–2499g | 3 (75.0) | 1 (25.0) | 4 (0.9) | 2.39 (0.69–8.20) | |
| 2500–2999g | 46 (88.5) | 6 (11.5) | 52 (11.6) | 2.82 (1.60–4.98) | |
| 3000–3499g | 121 (64.7) | 66 (35.3) | 187 (41.8) | 2.06 (1.22–3.47) | |
| 3500–3999g | 71 (49.3) | 73 (50.7) | 144 (32.1) | 1.57 (0.91–2.70) | |
| 4000–4499g | 16 (31.4) | 35 (68.6) | 51 (11.4) | 1.0 | |
| ≥4500g | 1 (10.0) | 9 (90.0) | 10 (2.2) | 0.32 (0.04–2.40) | |
GA: gestational age; PR: prevalence ratio; 95% CI: 95% confidence interval.
Statistically significant differences were found in respect to cord blood volume for gestational age>38 weeks (p-value=0.001), cesarean delivery (p-value<0.001) and birth weight>3500g (p-value<0.001).
A smaller volume of umbilical cord blood was collected when the gestational age was below 37 weeks and 6 days compared with gestational ages between 38 and 39 weeks and 6 days. Vaginal route of delivery was associated with a smaller volume of cord blood when compared to cesarean delivery. Newborn weight between 2500 and 3499g was associated with lower volume of umbilical cord blood when compared with infants weighing 4000–4999g. The prevalence of lower volume was approximately two times higher than the reference value.
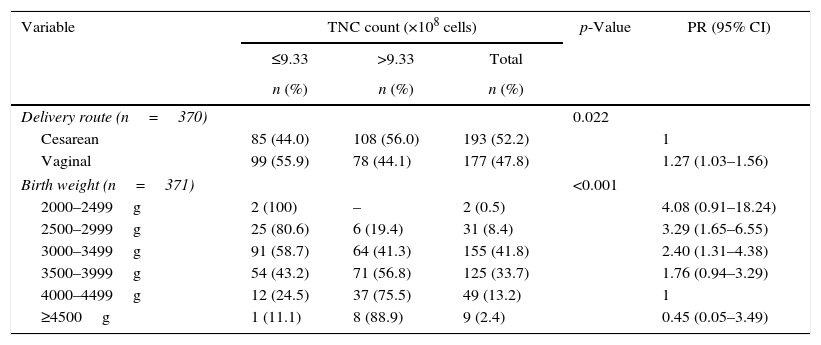
The comparison between maternal and fetal characteristics and TNC count is presented in Table 2.
Maternal and fetal characteristics and total nucleated cell count.
| Variable | TNC count (×108 cells) | p-Value | PR (95% CI) | ||
|---|---|---|---|---|---|
| ≤9.33 | >9.33 | Total | |||
| n (%) | n (%) | n (%) | |||
| Delivery route (n=370) | 0.022 | ||||
| Cesarean | 85 (44.0) | 108 (56.0) | 193 (52.2) | 1 | |
| Vaginal | 99 (55.9) | 78 (44.1) | 177 (47.8) | 1.27 (1.03–1.56) | |
| Birth weight (n=371) | <0.001 | ||||
| 2000–2499g | 2 (100) | – | 2 (0.5) | 4.08 (0.91–18.24) | |
| 2500–2999g | 25 (80.6) | 6 (19.4) | 31 (8.4) | 3.29 (1.65–6.55) | |
| 3000–3499g | 91 (58.7) | 64 (41.3) | 155 (41.8) | 2.40 (1.31–4.38) | |
| 3500–3999g | 54 (43.2) | 71 (56.8) | 125 (33.7) | 1.76 (0.94–3.29) | |
| 4000–4499g | 12 (24.5) | 37 (75.5) | 49 (13.2) | 1 | |
| ≥4500g | 1 (11.1) | 8 (88.9) | 9 (2.4) | 0.45 (0.05–3.49) | |
TNC: total nucleated cell; PR: prevalence ratio; 95% CI: 95% confidence interval.
Vaginal route of delivery was associated with a lower TNC count than cesarean delivery. Birth weights between 2500 and 3499g were associated with lower TNC counts compared to those between 4000 and 4499g, which were at least 2.4 times greater.
There were no statistically significant differences between quality markers – blood volume, TNC count and percentage of CD34+.
DiscussionCord blood is increasingly emerging as an alternative source of stem cells to the bone marrow for transplants, as it has a good number of hematopoietic progenitor cells with potential for reconstitution. These cells are essential for the treatment of some hematologic neoplasias.
In this analysis, other factors considered important, such as alcohol intake and smoking during pregnancy were not studied because this information did not exist in the patient's records.
In this study, vaginal route was the most common form of delivery, similar to the study by Rosenau et al.4 that investigated 1549 units of umbilical cord blood, of which 75.27% were collected after vaginal delivery; again similar to this study 52.03% of the newborns were male.4 According to Omori et al.,10 69.8% of the donors were primiparous. This fact might indicate a tendency of young people to donate umbilical cord blood as most of these women were young and 41.4% had no other child. This proves the importance of further research involving umbilical cord blood and the factors that influence the quality.
The mean birth weight of the newborn, according to a retrospective analysis by Mancinelli et al.,11 was 3390g and therefore similar to this study.
According to the results, the gestational age only positively influenced the volume of blood; the greater the gestational age, the larger the volume of umbilical cord blood. Askari et al., in order to demonstrate which variables affect the three main quality parameters of umbilical cord blood, showed that a gestational age of over 40 weeks was a predictor for a larger volume of blood, similar to the current study.6 Shu-Hui Wen et al. demonstrated that gestational age was inversely proportional to the volume.4 Along with increasing gestational age, there is a physiological placental senescence, especially from 36 weeks of gestation onwards. This placental aging creates a reduced oxygen supply to the fetus, releasing vasoconstrictor substances that, on reaching the placenta, could reflect in a reduced blood volume available for collection at birth. In this study no significant differences were found between gestational age and other quality parameters (TNC and CD34+ cell counts). However, some authors found a positive relationship between gestational age and the CD34+ cell count.12–15 This information casts doubt on the hypothesis that placental aging and consequent reduction in blood supply to the fetus could provide smaller numbers of umbilical cord blood hematopoietic cells. Some authors believe that with advancing gestational age and consequently with placental aging, the fetus would become progressively hypoxic, resulting in defense mechanisms. These mechanisms would be responsible for the increases in the number of hematopoietic cells in umbilical cord blood.11
When the route of delivery was analyzed, it was seen that cesarean section was associated with higher TNC counts and greater blood volumes. This result has been reported by several studies.5,11,13,14,16 However, other authors associated vaginal delivery with higher TNC counts.4,5,17 One hypothesis for cesarean sections being responsible for a greater volume of blood collected from the umbilical cord blood is the fact that the newborn is placed above the placenta before cord clamping, possibly causing a downward flow of blood into the umbilical cord and consequently into the placental compartment. Another hypothesis is that cesarean delivery permits a fast manual extraction of the placenta thereby reducing the chance of blood clot formation.11
As expected, the weight of the newborn at birth presented a positive relationship with two laboratory parameters analyzed: the TNC count and blood volume. In this study, significantly higher TNC counts and volumes were associated with birth weights above 3500g. To reinforce these findings, other studies reported that greater birth weight positively influenced these parameters.5,11,12,17,18 Mancinelli et al., in order to determine which obstetrics factors influence the quality of umbilical cord blood, showed that a higher birth weight was associated with better quality of umbilical cord blood in relation to the three main variables: TNC and CD34+ counts and the blood volume.11 Other authors also reported that this factor was associated with increased CD34+ counts4,13,19; this was not confirmed in the current study. Thus the greater birth weight, with a consequent increase in placental volume, somehow leads to increased blood volume in the placenta. However, unlike the results in the literature, this reasoning does not explain the correlation between placental volume according to birth weight and the number of CD34+ cells.
Possibly, the determinant of the quality of the stem cells will be defined after their use in patients and will thus be evaluated in the clinical practice. The increasing applicability and development of technology should serve to highlight the results found in this study and in many other studies that deal with this subject.
ConclusionsA gestational age greater than or equal to 38 weeks, cesarean delivery and birth weight greater than or equal to 3500g positively influence the blood volume collected from the umbilical cord. Cesarean delivery and birth weight greater than or equal to 3500g also have positive correlations with the TNC count. None of the maternal-fetal characteristics interfere with the CD34+ cell count.
Conflicts of interestThe authors declare no conflicts of interest.