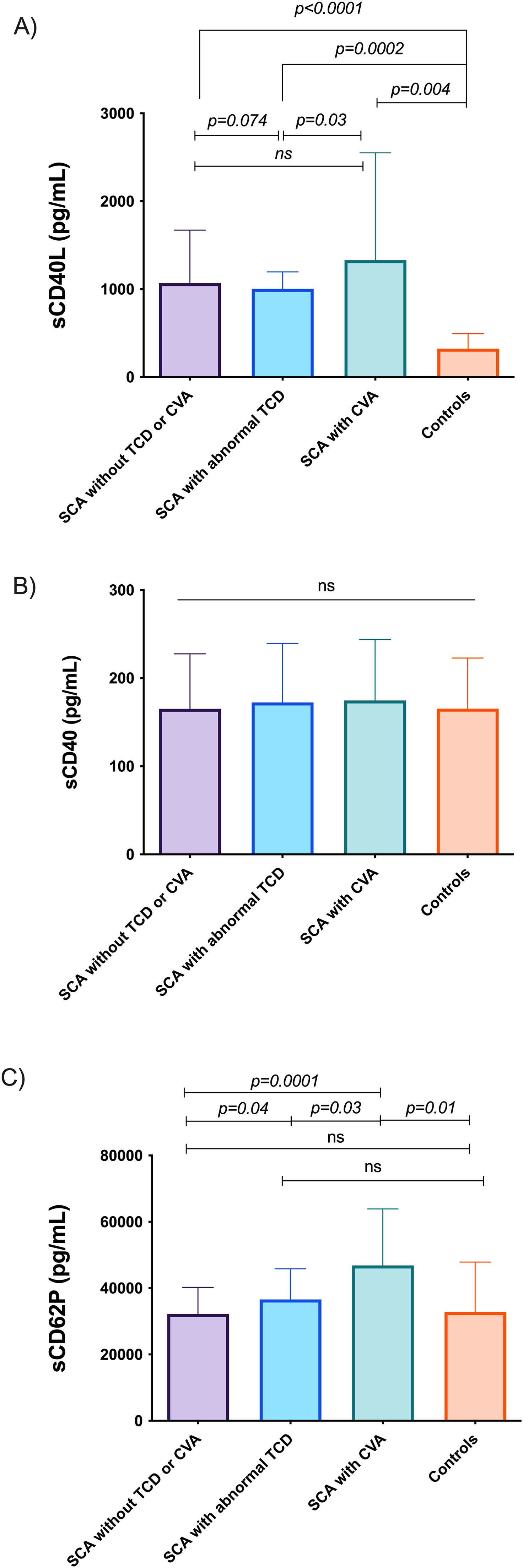
Serum levels of sCD40L, sCD40 and sCD62P were evaluated in sickle cell anemia (SCA) patients aged between 2 and 16 years with normal transcranial Doppler (TCD) and no stroke (G1, n = 24); in SCA patients with abnormal TCD (G2, n = 16); in SCA patients with a previous history of stroke (G3, n = 8), and; in healthy controls (aged 2 to 13 years; n = 26).
ResultsThe levels of sCD40L were significantly higher in the G1, G2 and G3 groups, compared to controls (p = 0.0001, p < 0.0002 and p = 0.004, respectively). Among patients with SCA, higher levels of sCD40L were found in the G3 group, compared to the G2 group (p = 0.03). In the sCD62P analysis, high levels in G3, compared to G1 (p = 0.0001), G2 (p = 0.03) and G4 (p = 0.01), and G2 also had high levels, compared to G1 (p = 0.04). The G1 patients had a higher sCD40L/sCD62P ratio, compared to G2 (p = 0.003) and controls (p < 0.0001). The sCD40L/sCD40 ratios were higher in G1, G2 and G3, compared to controls (p < 0.0001, p = 0.008 and p = 0.002, respectively).
ConclusionIt was concluded that the combination of TCD abnormality, associated with levels of sCD40L and sCD62P, may contribute to a better assessment of the risk for stroke in pediatric SCA patients.
Favor pedir para os autores corrigirem esta frase, pois NÃO é uma sentença completa em inglês: Our data suggest that decreased values of the s[LSM1] CD40L/sCD62P ratio involving two inflammatory mediators produced in platelet activation, being unprecedented in the literature.
Stroke is a severe neurologic complication of sickle cell anemia (SCA) and transcranial Doppler (TCD) screening is an important tool in predicting the risk of overt stroke.1-3 The TCD measures the cerebral blood flow velocity and an abnormal TCD (defined by a time-averaged velocity > 200 cm/second) has been predictive of a 40% risk of overt stroke in children with SCA.1-3
Platelets are involved in the process of hemostasis and inflammation and play an important role in the innate and adaptive immune response.4,5 Platelets express a large amount of the CD40 ligand (CD40L), also called CD154, and P-selectin (CD62P), both membrane-bound and soluble forms (sCD40L/sCD154 and sCD62P).4,5 The CD40 is presented on antigen-presenting cells (APCs), such as dendritic cells, macrophages and B lymphocytes and vascular endothelium. The CD40L (or CD154) are present on T-lymphocytes and on 95% of the circulating platelets4,5 and CD40-CD40L interactions are essential for the activation of APCs by cell-cell contact and the activation of endothelium.4,5
These proinflammatory molecules associated with platelets have been considered as markers of platelet activation in autoimmune diseases, chronic inflammatory diseases and cancer.6-8 However, the potential of these molecules as risk biomarkers for stroke in children with SCA is not described in the literature. Therefore, the investigation of these inflammatory mediators in peripheral blood is important for the early identification of patients at risk for stroke, avoiding severe and debilitating complications associated with SCA. Our data suggest that decreased values of the sCD40L/sCD62P ratio involving two inflammatory mediators produced in platelet activation, being unprecedented in the literature.
ObjectiveThis study aims to examine the relationship between sCD40L, sCD40 and sCD62P in peripheral blood of children and adolescents with SCA and an abnormal TCD.
MethodsSubjectsThis study included 48 patients with SCA, aged 2 to 16 years, followed at the Centro de Hematologia e Hemoterapia de Alagoas (HEMOAL) in Brazil, and the study was conducted at the Translational Research Laboratory Prof. C. A. Hart of the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP) in Recife, Brazil. The exclusion criteria for the patients included the use of hydroxyurea, blood components transfusion 21 days prior to the collection of the blood sample, infections in the last 30 days, chronic use of immunosuppressive drugs, previous diagnosis of primary or secondary immunodeficiency and gestation.
The control group included 26 healthy subjects, aged 2 to 16 years, undergoing outpatient elective surgery at the Hospital das Clínicas da Universidade Federal de Alagoas (UFAL) in Brazil.
All patients with SCA underwent TCD examination to evaluate cerebral blood flow velocity in the intracranial arteries using the Doppler, Probe 2 Mhz model, Ezdap, Germany. The time averaged maximum mean velocity (TAMM) was measured in the middle cerebral artery and internal carotid artery and a TCD ≥ 200 cm/s was considered abnormal. Patients with abnormal TCD with velocities below 200 cm/s (low conditional 171 – 184 cm/s and high conditional 185 – 199 cm/s) were not included in the study due to lack of data in the patient charts. Patients with SCA were divided into three groups: (G1) SCA, with normal TCD and no stroke (n = 24); (G2) SCA, with abnormal TCD and no stroke (n = 16), and; (G3) SCA, with a previous history of stroke (n = 8). Group 4 (G4) comprised healthy controls (n = 26).
The study was approved by the IMIP Research Ethics Committee and, after written informed consent had been provided by the parents of patients and controls, clinical data and blood samples were collected
Measurement of sCD40, sCD40L and CD62P and hematological parametersPeripheral blood collection was drawn in K2 EDTA tubes for the measurement of hematological parameters, sCD40, sCD40L and sCD62P. Blood samples of the patients were collected after performing the TCD and prior to the initiation of the hypertransfusion regimen. The dosages of hemoglobin (g/dL), reticulocytes (%), leukocytes (x 103/µL), neutrophils (x 103/µL), lymphocytes (x 103/µL) and platelets (x 103/µL) were performed in the automated cell counter (Cell-Dyn Ruby – Abbott Diagnostics, CA, USA).
The concentrations of sCD40 and sCD40L were measured in platelet-free plasma by the Enzyme-Linked Immunosorbent Assay (ELISA) using the Human sCD40 and sCD40L Human Platinum ELISA Kit (eBioscience, USA), respectively, according to the manufacturer's instructions. Values were expressed in pg/mL.
The concentration of sCD62P was measured in platelet-free plasma by flow cytometry (BD™ FACSVerse – BD Biosciences, CA, USA), using the BD™ CBA Human Soluble CD62P (P-Selectin) Flex Set, according to the manufacturer's instructions. The data were analyzed using the FCAP Array software (BD Biosciences, CA, USA) and the values were expressed in pg/mL.
Statistical analysisContinuous variables are represented as mean and standard deviation (SD) or median and interquartile range (IQR). The Shapiro-Wilk normality test was used to assess normality. Continuous variables were compared by the Kruskal-Wallis or ANOVA tests. The Spearman's (nonparametric) correlation coefficient was used for correlation analysis and interpreted as follows: 0.0 to 0.3, negligible; 0.3 to 0.5, weak; 0.5 to 0.7, moderate; 0.7 to 0.9, strong, and; 0.9 to 1.0, very strong. P-values < 0.05 were considered significant. The data analysis was performed in the GraphPad Prism v7.0 (GraphPad Software, San Diego, CA).
ResultsPatients with SCA (groups G1, G2 and G3) had significantly higher median values of sCD40L (p < 0.0001, p = 0.0002 and p = 0.004, respectively) than healthy controls (group G4; Figure 1A). Among patients with SCA, significantly higher levels of sCD40L were observed in patients in group G3 (median 1.330 pg/mL) when compared to patients in-group G2 (median 1.003 pg/mL, p = 0.03; Figure 1A). The comparison of sCD40 levels showed no significant difference between groups (Figure 1B).
Levels of sCD62P were higher in group G3 patients, when compared to groups G1, G2 and G4 (p = 0.0001, p = 0.03, p = 0.01, respectively), and group G2 patients had higher levels, compared to group G1 patients (p = 0.04; Figure 1C).
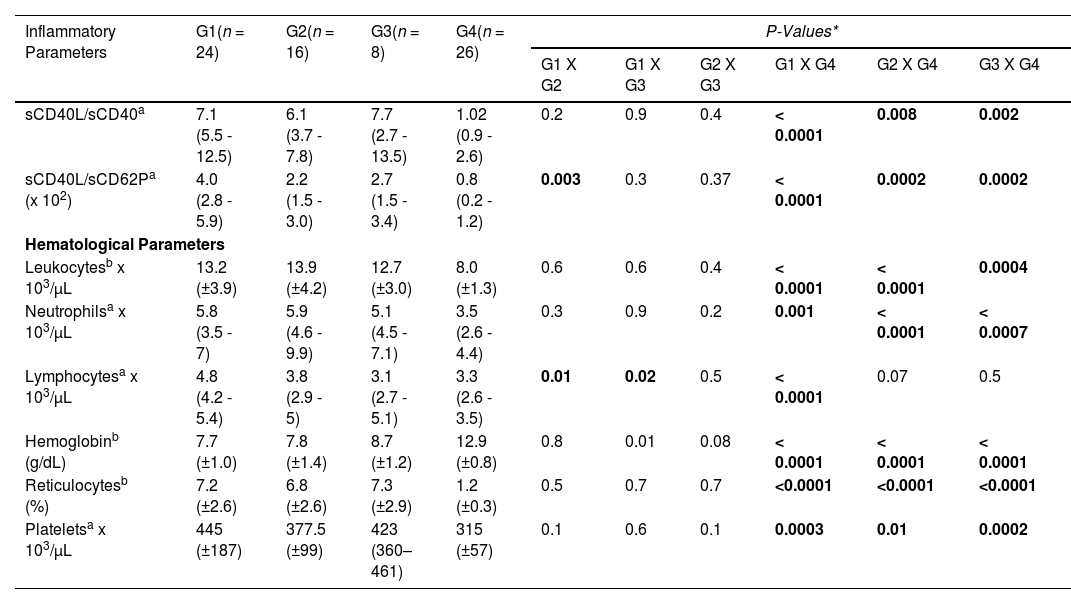
The sCD40L/sCD40 ratios were increased 7-fold, 6-fold, and 7.4-fold in the groups G1, G2 and G3, respectively, compared to healthy controls (p < 0.0001, p = 0.008 and p = 0.002, respectively). However, no significant differences were observed between groups G1, G2 and G3. Analysis of the sCD40L/sCD62P ratio showed that group G1 patients had a higher ratio, compared to group G2 patients (p = 0.003) and healthy controls (p < 0.0001). No significant differences were observed between group G3 and groups G1 and G2, as shown in Table 1.
Inflammatory and hematological parameters of patients with sickle cell anemia according to clinical forms of the disease and control group.
| Inflammatory Parameters | G1(n = 24) | G2(n = 16) | G3(n = 8) | G4(n = 26) | P-Values* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 X G2 | G1 X G3 | G2 X G3 | G1 X G4 | G2 X G4 | G3 X G4 | |||||
| sCD40L/sCD40a | 7.1 (5.5 - 12.5) | 6.1 (3.7 - 7.8) | 7.7 (2.7 - 13.5) | 1.02 (0.9 - 2.6) | 0.2 | 0.9 | 0.4 | < 0.0001 | 0.008 | 0.002 |
| sCD40L/sCD62Pa (x 102) | 4.0 (2.8 - 5.9) | 2.2 (1.5 - 3.0) | 2.7 (1.5 - 3.4) | 0.8 (0.2 - 1.2) | 0.003 | 0.3 | 0.37 | < 0.0001 | 0.0002 | 0.0002 |
| Hematological Parameters | ||||||||||
| Leukocytesb x 103/µL | 13.2 (±3.9) | 13.9 (±4.2) | 12.7 (±3.0) | 8.0 (±1.3) | 0.6 | 0.6 | 0.4 | < 0.0001 | < 0.0001 | 0.0004 |
| Neutrophilsa x 103/µL | 5.8 (3.5 - 7) | 5.9 (4.6 - 9.9) | 5.1 (4.5 - 7.1) | 3.5 (2.6 - 4.4) | 0.3 | 0.9 | 0.2 | 0.001 | < 0.0001 | < 0.0007 |
| Lymphocytesa x 103/µL | 4.8 (4.2 - 5.4) | 3.8 (2.9 - 5) | 3.1 (2.7 - 5.1) | 3.3 (2.6 - 3.5) | 0.01 | 0.02 | 0.5 | < 0.0001 | 0.07 | 0.5 |
| Hemoglobinb (g/dL) | 7.7 (±1.0) | 7.8 (±1.4) | 8.7 (±1.2) | 12.9 (±0.8) | 0.8 | 0.01 | 0.08 | < 0.0001 | < 0.0001 | < 0.0001 |
| Reticulocytesb (%) | 7.2 (±2.6) | 6.8 (±2.6) | 7.3 (±2.9) | 1.2 (±0.3) | 0.5 | 0.7 | 0.7 | <0.0001 | <0.0001 | <0.0001 |
| Plateletsa x 103/µL | 445 (±187) | 377.5 (±99) | 423 (360–461) | 315 (±57) | 0.1 | 0.6 | 0.1 | 0.0003 | 0.01 | 0.0002 |
SCA: sickle cell anemia; TCD: Transcranial Doppler; G1: SCA, normal TCD and no stroke; G2: SCA, abnormal TCD and no stroke; G3: SCA and stroke; G4: Controls.
In this study, we analyzed the relationship between the levels of the soluble molecules sCD40L, sCD40 and sCD62P in the peripheral blood of children and adolescents with SCA and an abnormal TCD. The combination of TCD abnormality and mediators, such as sCD40L and sCD62P, could provide a better assessment of the risk for stroke in pediatric SCA patients.
Our data corroborate the findings from Majumdar et al., which showed a significant increase in platelet activation through of the dosage of sCD40L levels in patients with SCA and stroke or abnormal TCD and intracranial stenosis.9 Elevated levels of sCD40L were also observed by Lee et al., 2006, in a study with 45 patients with SCA, including eight patients with vaso-occlusion and 37 patients in the non-crisis steady state.10 This suggests an important role of platelet-derived inflammatory mediators in the process of chronic inflammation and thrombotic activity, which are common features of SCA.10
Higher levels of sCD40L may reflect a high expression on the surface and a release of sCD40L by activated platelets,6 suggesting that sCD40L may represent a potential soluble biomarker of vascular injury and thrombus formation in patients with SCA and stroke. Studies show elevated sCD40L levels in acute myocardial infarction and chronic inflammatory diseases.6,9-15
Our results indicate that the soluble form of CD40 is not involved in chronic diseases and cerebrovascular disease in SCA. The CD40 is presented on antigen-presenting cells (APCs), such as dendritic cells, macrophages and B lymphocytes and the vascular endothelium. The CD40L is present on T-lymphocytes and on 95% of the circulating platelets4,5 and CD40-CD40L interactions are essential for the activation of APCs by cell-cell contact and the activation of the endothelium.13
Kutlar et al. demonstrated that the increased expression of CD62P in the microvascular endothelium of patients with SCA represents an important role in mechanisms of acute vascular occlusion and in the chronic form can cause changes in the blood flow.16 Our data show significantly higher levels of sCD62P in group G2 patients, compared to group G1 patients. Abnormal TCD refers to increased blood flow velocity through the large cerebral arteries,16 which may lead to increased platelet activation, even without vascular injury, resulting in elevated sCD62P levels. Thus, our findings indicate that sCD62p may be a possible biomarker of installed cerebrovascular disease or pre-disease in patients with SCA.
Patients with SCA showed higher absolute values of leukocytes, neutrophils, reticulocytes and platelets and lower values of hemoglobin, compared to healthy controls. These findings corroborate the continuous hemolytic condition that occurs in patients with SCA, which is independent of the complications of the disease. These complications are more common in the vaso-occlusion, associated with a reactive bone marrow and evidenced by the high reticulocytosis found in these patients.17,18
A study on inflammatory platelet markers and increased risk of thrombosis in patients with Coronavirus Disease (COVID-19), in which high levels of P-selectin were found, demonstrated that platelet hyperactivity may be associated with a higher incidence of thrombotic phenomena in this group of patients.19 Similar behavior was observed in our results in patients with sickle cell anemia and cerebrovascular disease. In this sense, knowledge about the pathways that lead to the development of platelet hyperactivity contributes to the understanding of thrombotic risk and helps to optimize the prevention of stroke, in addition to enhancing treatment strategies.
Particular observation of the high frequency of thrombotic events in patients with COVID-19 is also consistent with a P-selectin-mediated activation of intravascular coagulation.20 Anti-P-selectin monoclonal antibodies, such as crizanlizumab, recently approved for use in patients with sickle cell anemia, revealed a decrease in painful crises, thus contributing to the importance of P-selectin in the pathophysiology of cerebral thrombotic events in these patients.21
In this manner, a similar study on patients with acute myocardial infarction showed that the association of two inflammatory mediators, troponin and sCD40L, increases the accuracy of the prediction of infarction.14
ConclusionTherefore, the combination of the TCD abnormality with possible mediators, such as sCD40L and sCD62P, could provide a better assessment of risk of stroke in pediatric SCA patients. Moreover, further studies evaluating these molecules on the cell surface and correlating them with the soluble forms are needed to better clinical application, as these molecules could be a target for the development of therapeutic strategies for SCA.
The authors thank the patients and their families for their contribution and Camilo Vieira for his help with the TCD exam. This study was supported by grants from the Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE).