Systemic and pulmonary coagulopathy and inflammation are important characteristics of transfusion-related acute lung injury (TRALI). Whether microparticles that accumulate in transfused red blood cell concentrates (RBCs) have proinflammatory and procoagulant potential and contribute to adverse reactions of RBC transfusions is unclear.
AimTo investigate the ability of microparticles in stored RBCs to promote thrombin generation and induce human pulmonary microvascular endothelial cell (HMVEC) activation and damage.
MethodsThe number and size of microparticles were determined by flow cytometric and nanoparticle tracking analyses, respectively. Thrombin generation and the intrinsic coagulation pathway were assayed by a calibrated automated thrombogram and by measuring activated partial thromboplastin time (aPTT), respectively. The expression of ICAM-1 and the release of cytokines by endothelial cells were detected by flow cytometric analyses. HMVEC damage was assessed by incubating lipopolysaccharide-activated endothelial cells with MP-primed polymorphonuclear neutrophils (PMNs).
ResultsThe size of the microparticles in the RBC supernatant was approximately 100–300 nm. Microparticles promoted thrombin generation in a dose-dependent manner and the aPTT was shortened. Depleting microparticles from the supernatant of RBCs stored for 35 days by either filtration or centrifugation significantly decreased the promotion of thrombin generation. The expression of ICAM-1 on HMVECs was increased significantly by incubation with isolated microparticles. Furthermore, microparticles induced the release of interleukin-6 (IL-6) and interleukin-8 (IL-8) from HMVECs. Microparticles induced lipopolysaccharide-activated HMVEC damage by priming PMNs, but this effect was prevented by inhibiting the PMNs respiratory burst with apocynin.
ConclusionMicroparticles in stored RBCs promote thrombin generation, HMVEC activation and damage which may be involved in TRALI development.
Red blood cell (RBC) microparticles (MPs) are membrane-bound vesicles derived from RBCs under physiological or pathological conditions and are considered a RBC storage lesion.1-4 During RBC storage, MPs may be released from several types of cells in red cell products with RBC MPs accounting for the majority of released MPs.5,6 Research has indicated that the procoagulant and proinflammatory activity of RBC MPs may be relevant to hemostasis, inflammation and vascular reactions.7-9 MPs rich in phospholipids contribute to the coagulant pathway. 10 These MPs may participate in the coagulation pathway, although whether tissue factor (TF) is present in MPs is uncertain.11 MPs that interact with endothelial cells or monocytes may induce the release of interleukin-1β (IL-1β), interleukin-6 IL-6, interleukin-8 IL-8, TNF-α, CXCL-8 and other proinflammatory cytokines. 12-14 Since MPs contain phospholipids and hemoglobin, they could also significantly affect nitric oxide (NO) bioavailability. 15 Therefore, MPs may play an important role in hemolysis or endothelium-activated clinical conditions such as sickle cell anemia, thalassemia, hereditary polycythemia and acute graft-versus-host disease.16,17
Transfusion-related acute lung injury (TRALI) is a subcategory of acute respiratory distress syndrome (ARDS). The special additional diagnostic requirement for TRALI is occurrence within six hours of blood transfusion. Systemic and pulmonary inflammation, coagulopathy and increased vascular and endothelial permeability are characteristics of this condition. Previous studies have indicated that the transfusion of stored RBC supernatant or isolated RBC MPs cause inflammation and coagulation in the lung.18-20 For example, the transfusion of RBC supernatant induced acute lung injury in mice as the second event of two-event mouse TRALI. 21,22 RBC MPs are believed to be potential mediators of non-antibody-mediated TRALI.23 Similarly, our investigations reported that RBC MPs primed the polymorphonuclear neutrophil (PMN) respiratory burst and caused TRALI in mice.24,25
To determine the proinflammatory and procoagulant potential of MPs in stored RBCs and their roles in the pathological mechanism of TRALI, we intend to isolate MPs from stored RBCs and investigate the effects of these MPs on thrombin generation, and HMVEC activation and damage.
Materials and methodsSample preparationWhole blood was stored in a polyvinyl chloride collection bag system (Shanghai Transfusion Technology Co. Ltd., Shanghai, China) containing an anticoagulant, citrate dextrose solution B (ACD-B) (Shanghai Transfusion Technology Co. Ltd.). After the blood was stored overnight, the plasma was separated via centrifugation at 3820 × g for 20 min and mannitol-adenine-phosphate (MAP) additive solution (Shanghai Transfusion Technology Co., Ltd., Shanghai, China) was then added to produce a final hematocrit level of 50–65% to maintain RBC vitality for 35 days. Samples were drawn from stored RBCs at regular intervals of seven days using a sterile tubing welder (Terumo BCT, Lakewood, CO, USA).
MPs were isolated using the method previously described by Rubin et al. and Jy et al., with some modifications.26,27 Briefly, samples of stored RBCs were centrifuged at room temperature at 1850 × g for 20 min and the supernatant was collected; this process was then repeated. The supernatant was centrifuged at 4 °C and 20,000 × g for one hour and the resulting pellets were resuspended in phosphate-buffered saline (PBS; pH 7.2, Gibco, Life Technologies, Grand Island, NY, USA) supplemented with 0.3% citric acid (Sigma, St. Louis, MO, USA). After the MPs were washed twice, they were prepared as a 10-fold concentrated suspension in PBS buffer.
To obtain MP-depleted supernatants, RBC supernatants were processed either by filtration or by centrifugation. Filtration was performed with a 0.1-μm polyvinylidene Fluoride membrane filter which is supposed to remove 99.9% MPs from RBC supernatants (Millipore Ltd., Darmstadt, Germany). The pre- and post-filtration supernatants were used in the corresponding experiments. For processing by centrifugation, the supernatants were centrifuged at 4 °C and 20,000 × g for one hour and collected.
Microparticle analysisThe distribution of MPs in the RBC supernatants was assayed by nanoparticle tracking analysis (NanoSight LM10, Malvern Panalytical, UK). Three types of MPs in stored RBCs were counted by flow cytometric analysis. 28 RBC MPs, platelet microparticles (PMPs) and leukocyte microparticles (LMPs) in the RBC supernatant were double-labeled with a phycoerythrin (PE)-conjugated mouse anti-human CD235a (IgG1, clone HIR2) monoclonal antibody (BD Biosciences, San Jose, CA, USA), an APC-conjugated mouse anti-human CD61 antibody (IgG1, clone VI-PL2) or an APC-conjugated mouse anti-human CD45 (IgG1, clone HI30) antibody, respectively, combined with 10 µM carboxyfluorescein succinimidyl ester (CFSE - Life Technologies Corporation, OR, USA). Before flow cytometric analysis (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ, USA), 500 μL of the labeled sample was pipetted into a BD TruCOUNT tube (BD Biosciences) and gently vortexed. The events positive for fluorescence of the respective antibody and CFSE were gated and counted. In addition, 5000 bead events were acquired. The number of MPs was calculated according to the manufacturer's instructions for the BD TruCOUNT tube.
Thrombin generation testsThrombin generation tests (TGTs) were performed by a calibrated automated thrombogram assay. Tests were performed with a plate reader (Fluoroskan Ascent, Thermo Labsystems, Helsinki, Finland) and computer software (Thrombinoscope, Thrombinoscope BV, Maastricht, the Netherlands) as described by the manufacturers. Briefly, 20 μL of the sample or thrombin calibrator (Thrombinoscope BV), 60 μL of MP-depleted plasma and 20 μL of platelet-rich plasma (PRP) reagent (Thrombinoscope BV) containing 1 pmol/L TF and a minimal amount of phospholipids were added to a 96-well plate in duplicate. After incubation, 20 μL of fluorescent calcium reagent (Thrombinoscope BV) was added automatically. Thrombin generation was recorded by the computer software. The data are expressed as nanomoles of thrombin generated per unit time (ETP, nM/min), peak, lag time (min) and time to peak (min).
Partial thromboplastin time/activated partial thromboplastin time assaysThe partial thromboplastin time (PT) and aPTT were assayed with an ACL7000 hemostasis testing system (Instrumentation Laboratory, Bedford, MA 01730, USA) combined with a HemosIL RecombiPlasTin 2G Kit or a HemosIL SynthASil Kit (Instrumentation Laboratory, Bedford, MA 01730, USA) for PT and aPTT, respectively. Briefly, the tray was placed in the instrument, the intended assay component (PT-fibrinogen or aPTT) was selected, the required reagent was added to the reagent cup, and the sample or calibration plasma was added to the indicated cup on the sample tray. The result is reported in units of seconds or percent activity.
Expression of ICAM-1 on human pulmonary microvascular endothelial cells (HMVECs)HMVECs (Pricell, Wu Han Biomedical & Technical Ltd., China) were grown to ≥90% confluence in 96-well plates. After the incubation of endothelial cells with MPs for 2, 6, 12 or 24 h, the cells were detached with 0.05% trypsin-EDTA (Gibco). To examine the expression of ICAM-1 on HMVECs, 1 × 106 cells were incubated with 10 μL of mouse anti-human CD54-PE antibody (BD, Biosciences, San Jose, CA, USA) in 100 μL of PBS for 30 min at room temperature. The cells were diluted to 600 μL with PBS before flow cytometric analysis. The mean fluorescence intensity of the HMVECs was recorded.
Release of the cytokines interleukin-6 and interleukin-8After incubation of HMVECs with MPs for 2, 6, 12 or 24 h, the medium was collected, centrifuged at 12,000 × g for five minutes to remove dead cells or debris, and stored at −80 °C. The levels of IL-6 and IL-8 were simultaneously determined using a human cytokine cytometric bead array (CBA Kit, BD Pharmingen) according to the instructions provided by the manufacturer. Cytokine bead staining was analyzed by flow cytometry, and the data were compiled with BD Biosciences CBA software.
Induction of human pulmonary microvascular endothelial cell (HMVEC) damage by microparticles in stored red blood cellsHMVEC damage was induced as described in our previous study.29 Briefly, HMVECs were grown to ≥90% confluence in 96-well plates. The cells were treated with 200 ng/mL lipopolysaccharide (LPS, Sigma-Aldrich) for six hours. PMNs were added to endothelial cells at an effector cell:target cell ratio of 10:1. After settling, the PMNs were exposed to MPs or buffer for 30 min. The numbers of viable cells were counted over a 1-mm2 surface area by fluorescence microscopy after staining with a LIVE/DEAD Cell Imaging Kit (Life Technologies Corporation, OR, USA). The PMN respiratory burst was inhibited by incubating PMNs with 300–1200 μM apocynin for 15 min at 37 °C before adding them to the HMVECs.
Statistical analysisFor the different experimental groups, the data are presented as means ± standard deviations (SDs) or standard errors of the mean (SEMs). To compare the differences among multiple groups, the 95% confidence intervals (95% CI) are included. One-way ANOVA was performed, followed by Tukey's multiple comparison test. A paired t-test was used to calculate differences between two groups. A p-value <0.05 was defined as statistically significant.
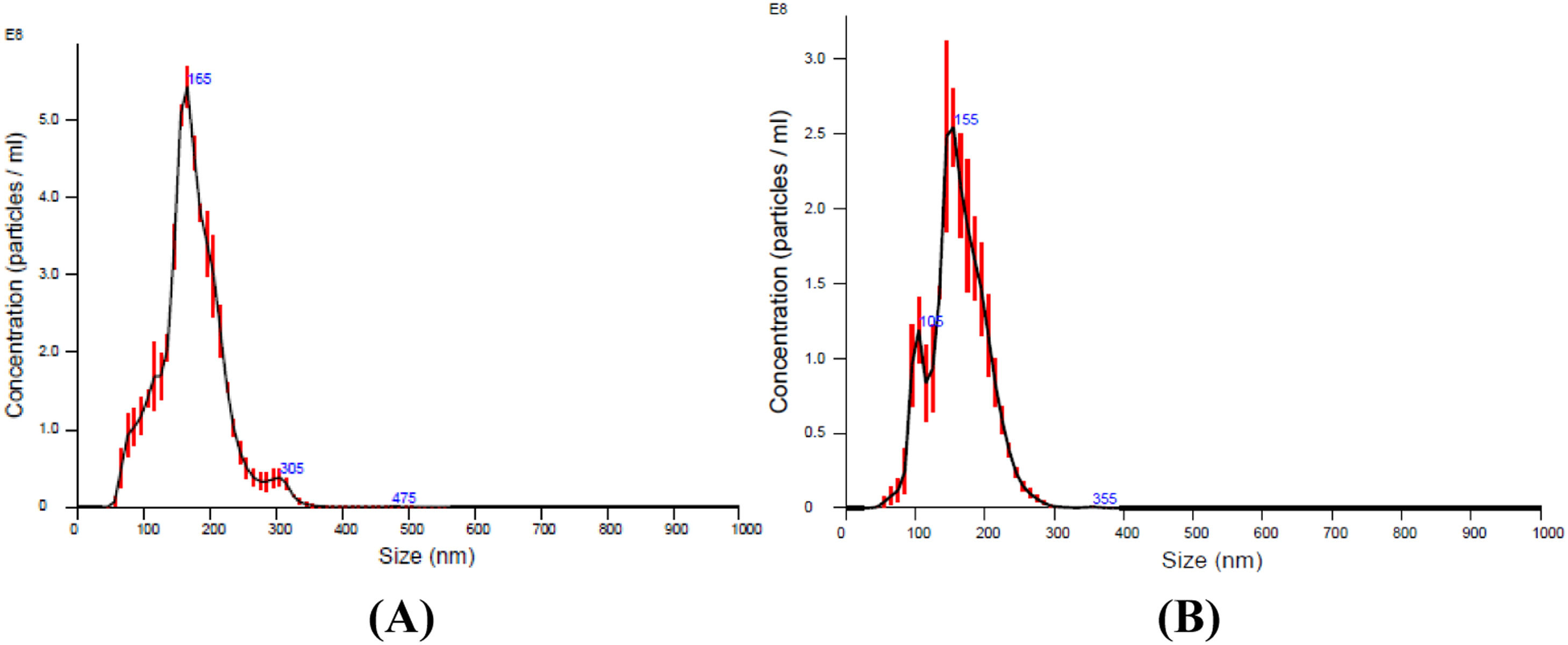
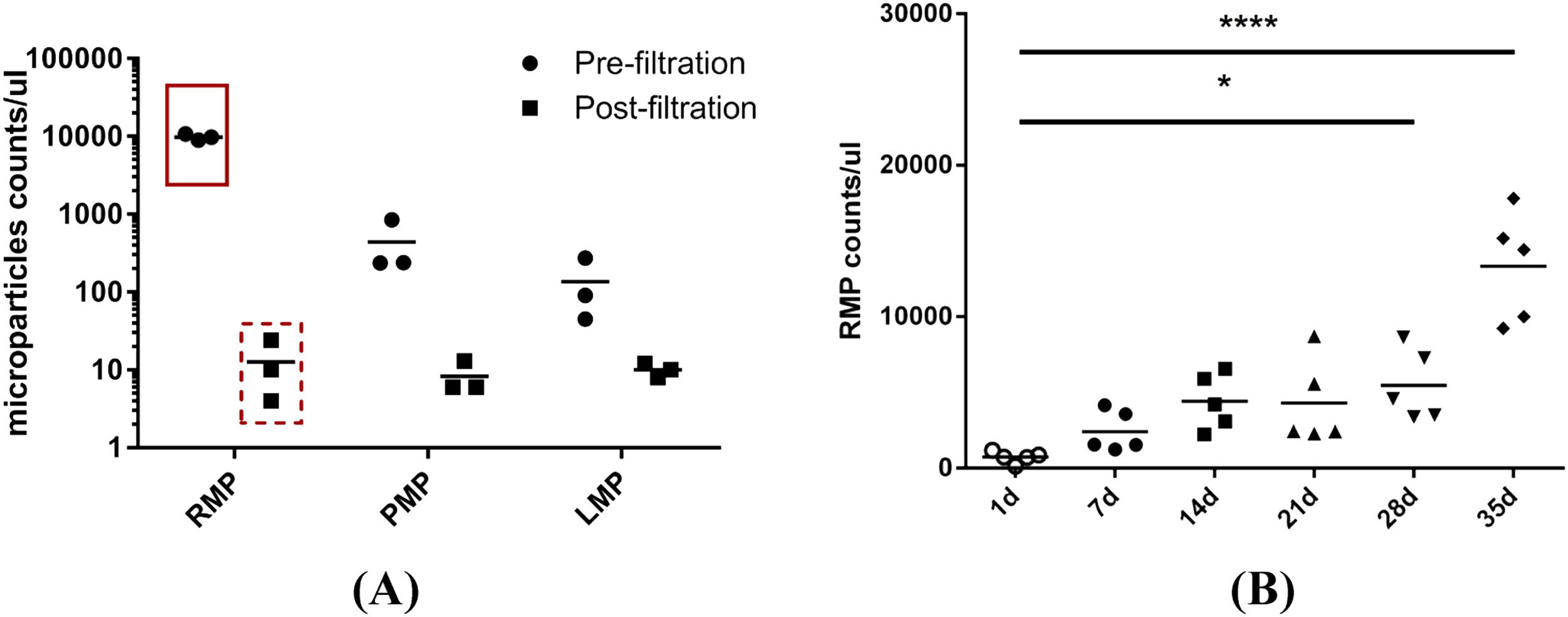
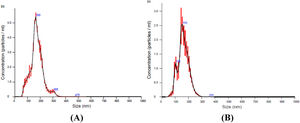
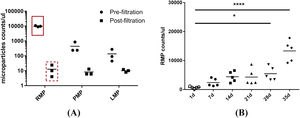
ResultsMicroparticles in stored red blood cellsThe results of the nanoparticle tracking analysis indicated that the size of the majority of the MPs in the RBC supernatant was approximately 100–300 nm. MPs were depleted significantly by filtration through a 0.1 µm filter (Figure 1A, B). Because non-leukoreduced RBCs were used in this study, three types of MPs in stored RBCs were counted by flow cytometric analysis. The results show that RBC MPs were the major MPs present (approximately 94.5%; Figure 2A) and that the RBC MP count increased significantly after RBCs were stored for 28 days (Figure 2B).
Nanoparticle tracking analysis of microparticles (MPs) in supernatant obtained from red blood cells (RBCs) centrifuged at 1850 × g for 20 min. (A) Microparticles in supernatant of RBCs stored for 35 days. (B) Microparticles in supernatant of RBCs stored for 35 days after filtration with a 0.1 μm filter.
Flow cytometric analysis of microparticles (MPs) in red blood cell (RBC) supernatant. (A) RBC MPs, platelet MPs and leukocyte MPs in RBC supernatant were double-labeled with mouse anti-human CD235a, mouse anti-human CD61 and mouse anti-human CD45 respectively, combined with 10 µM of carboxyfluorescein succinimidyl ester (CFSE). The amount of MPs in pre- and post-filtration of RBC supernatant (0.1 μm filter) stored for 35 days were counted with a BD TruCOUNT tube by flow cytometric analysis (n = 3). (B) RBC MPs accumulated in RBCs during storage for 35 days were double-labeled with mouse anti-human CD235a combined with 10 µM of CFSE and counted by the same method (n = 5). * p-value <0.05.
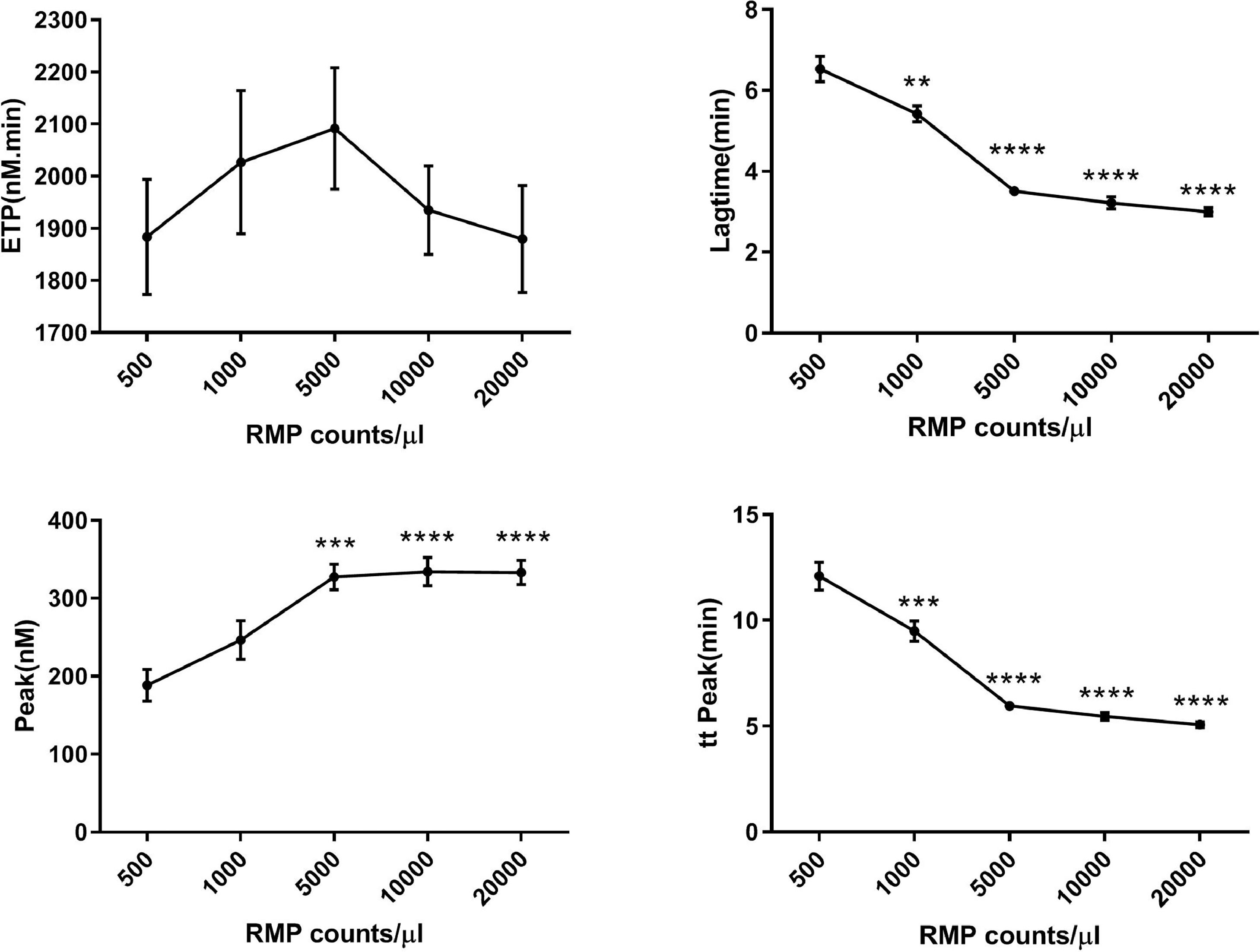
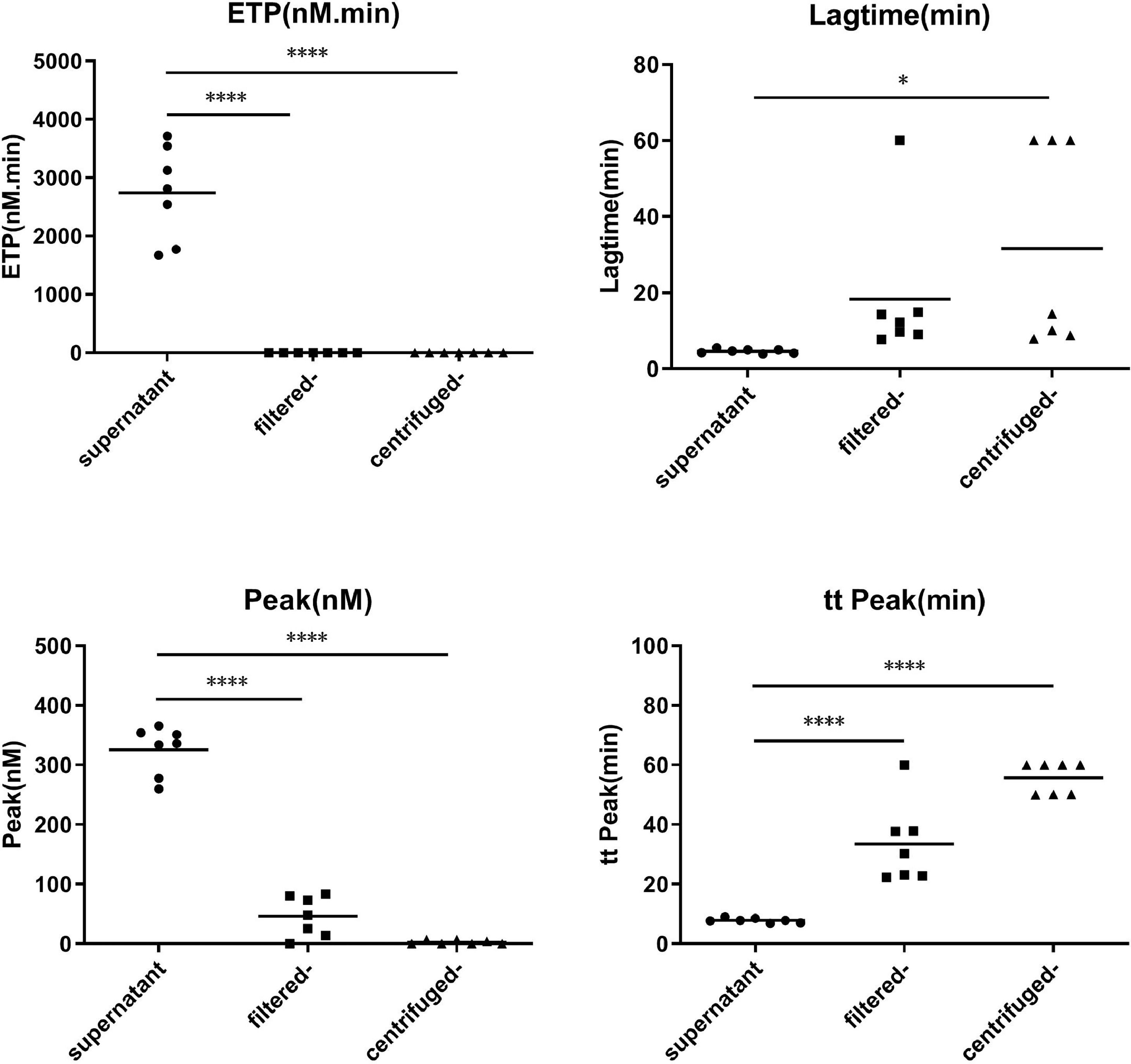
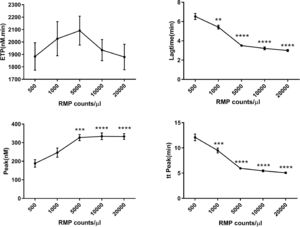
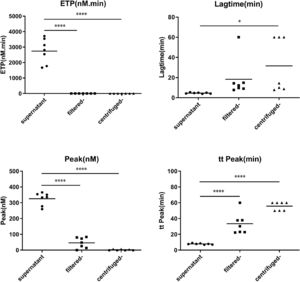
Concentrated MPs from RBCs stored for 35 days were serially diluted in MP-depleted fresh plasma. With increasing amounts of RBC MPs, the ETP and peak values increased, while the lag time and time to peak values decreased (Figure 3). These results indicated that the RBC MPs promoted thrombin generation in a dose-dependent manner from an RBC MP concentration of 500 to 5000 per microliter; no additional increase was observed with higher RBC MP concentrations. Depletion of MPs from the supernatant by either filtration or centrifugation reduced the amount of MPs and significantly decreased the potential of MPs to promote thrombin generation (p-value <0.05; Figure 4).
Calibrated automated thrombogram (CAT) assay showing that serially diluted red blood cell (RBC) microparticles (MPs) promote thrombin generation in plasma (n = 5). RBC supernatant was centrifuged at 4 °C and 20,000 × g for one hour and washed with phosphate-buffered saline (PBS) containing 0.3% citric acid. They were prepared as a 10-fold concentrated suspension in PBS buffer before dilution. Thrombin generation was recorded by computer software. The data are expressed as nanomoles of thrombin generated per unit time (ETP, nM/min), lag time (min), peak of the thrombin concentration (nM) and time to peak (min). * p-value <0.05,**p-value<0.01,***p<0.001,****p<0.0001; compared with the group of 500 RMP counts/μl.
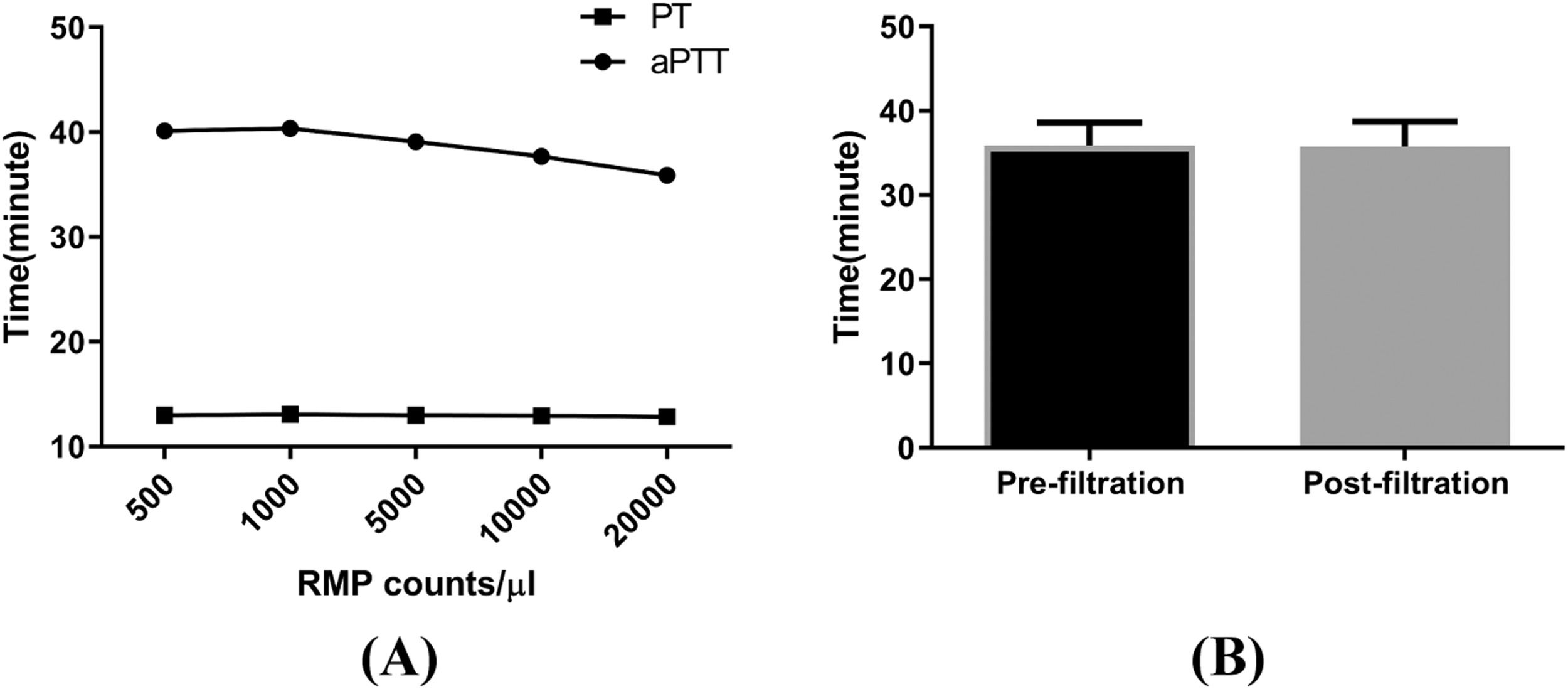
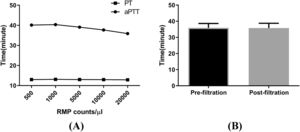
Although the PT was not affected, the aPTT decreased on adding concentrated MPs to MP-depleted plasma, and the effects exhibited dose dependency for RBC MP concentrations of 1000–20,000 per microliter. There was no difference in the aPTT between the filtered supernatant and the control supernatant (Figure 5).
Effects of microparticles (MPs) on partial thromboplastin time/activated partial thromboplastin time (PT/aPTT). The PT and aPTT were assayed with an ACL7000 hemostasis testing system. Samples were prepared by serially diluting red blood cell (RBC) MPs with platelet poor plasma. (A) Result of aPTT and PT (n = 7). (B) aPTT analysis for the pre- and post-filtration (0.1 μm filter) supernatant from RBCs stored for 35 days (n = 7). .
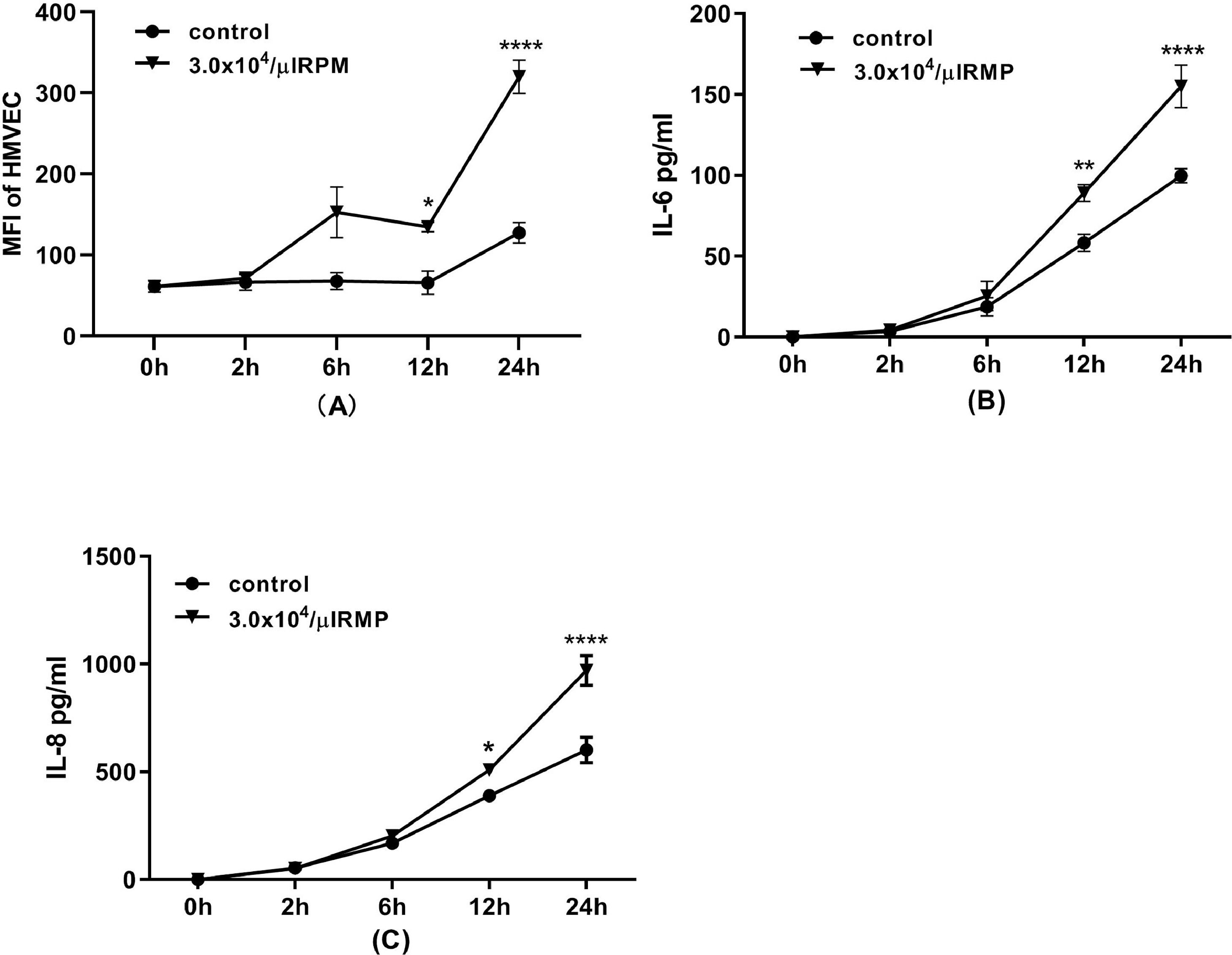
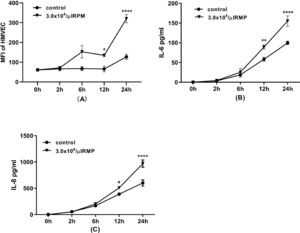
In this experiment, endothelial cells were incubated with MPs for 24 h. The expression of ICAM-1 on HMVECs was increased significantly after six hours and increased continuously until 24 h of incubation (Figure 6A). Furthermore, MPs in stored RBCs induced the release of the inflammatory cytokines IL-6 and IL-8 by HMVECs after 12 h of incubation (Figure 6B & C).
Flow cytometric analysis of human pulmonary microvascular endothelial cells (HMVECs) during 24 h of incubation. (A) ICAM-1 expression on HMVECs (n = 3). After the incubation of endothelial cells with microparticles (MPs) for 2, 6, 12 or 24 h, the cells were detached and labeled with mouse anti-human CD54 before flow cytometric analysis. Interleukin-6 (IL-6) (B) and interleukin-8 (IL-8) (C) released from HMVECs (n = 3). After incubation of HMVECs with MPs for 2, 6, 12 or 24 h, the medium was collected, centrifuged at 12,000 × g for five minutes to remove dead cells or debris. The levels of IL-6 and IL-8 were simultaneously determined using a human cytokine cytometric bead array (CBA Kit, BD Pharmingen) according to the instructions provided by the manufacturer. * p-value <0.05,**p-value<0.01,***p-value<0.001,****p-value<0.0001; compared with the contol group at same time point.
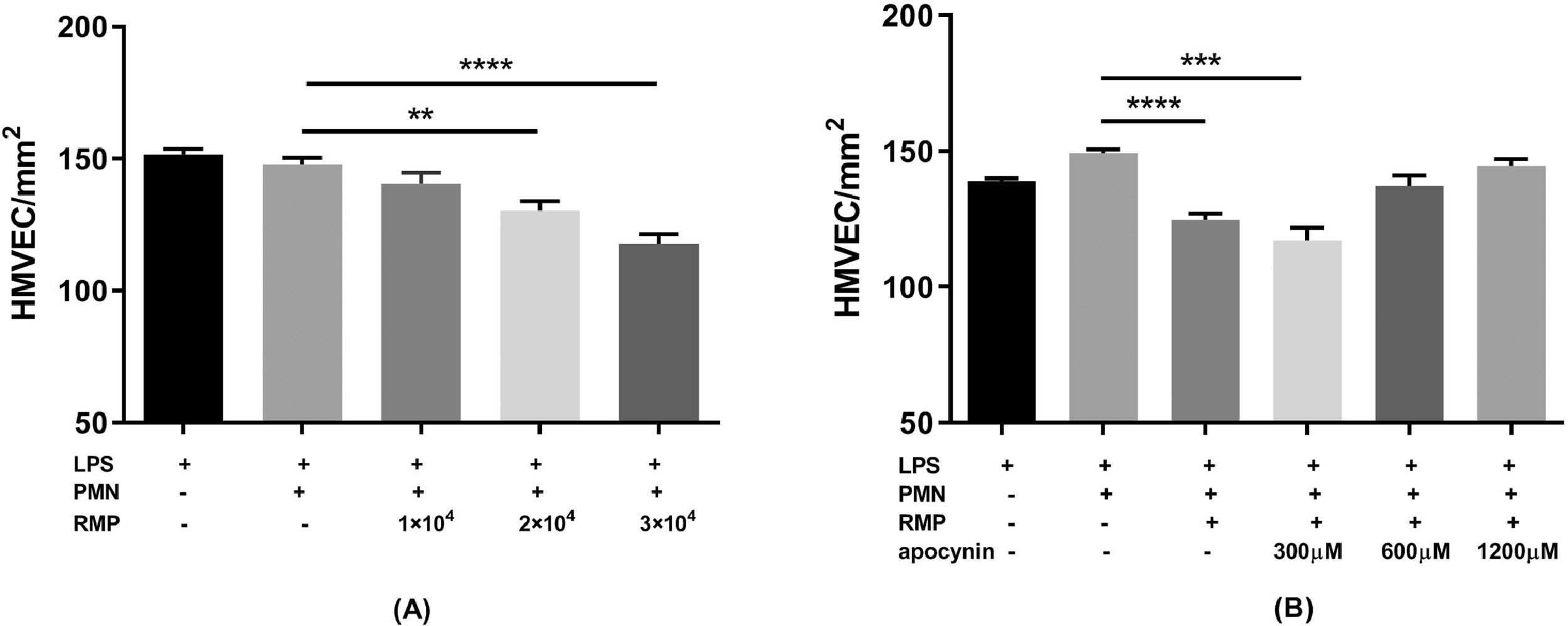
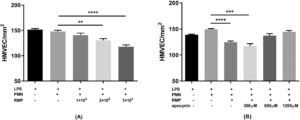
The PMN-mediated HMVEC damage indicated that RBC MPs could induce damage to LPS-treated HMVECs, but this damage was prevented by inhibiting the PMN respiratory burst with apocynin (Figure 7).
Red blood cell (RBC) microparticles (MPs) induced lipopolysaccharide (LPS)-activated human pulmonary microvascular endothelial cell (HMVEC) damage. The cells were treated with 200 ng/mL LPS for six hours. Polymorphonuclear neutrophils (PMNs) were added to endothelial cells at an effector cell:target cell ratio of 10:1. After settling, the PMNs were exposed to MPs or buffer for 30 min. The numbers of viable cells were counted over a 1-mm2 surface area by fluorescence microscopy after staining with a LIVE/DEAD Cell Imaging Kit. The PMN respiratory burst was inhibited by incubating PMNs with 300–1200 μM apocynin for 15 min at 37 °C before adding them to HMVECs. (A) Effect of serially diluted RBC MPs on HMVEC damage (n = 5). (B) Effect of treating PMNs with apocynin on RBC MP-induced HMVEC (3 × 104/μL) damage (n = 5). * p-value <0.05,**p-value<0.01,***p-value<0.001,****p-value<0.0001.
MPs may be released from various blood cells, including red cells, leukocytes and platelets. Non-leukocyte reduced RBCs are still used in developing countries. We detected three kinds of MPs that may occur in RBCs. The results showed that the majority of MPs (approximately 94.5%) in stored RBCs expressed a RBC-specific marker, CD235a. The depletion of MPs from the supernatant of stored RBCs by 0.1 µm filtration decreased its potential of promoting thrombin generation significantly and prolonged the clotting time. The study by Aung et al. reported that the depletion of phosphatidylserine (PS)-bearing MPs by 0.22-µm filtration did not reduce phospholipid-dependent procoagulant activity.30 In agreement with our result, it can be speculated that major procoagulant potential is caused by RBC supernatants which contain MPs smaller than 0.22 µm but bigger than 0.1 µm. The size of these MPs was very similar to that in the study of Rubin et al., which was reported as approximately 0.15 µm.26 The residual procoagulant potential in RBC supernatant may be from other forms of procoagulant phospholipids, such as bioactive lipids, exosomes, and microRNA.31
Thrombin generation involves both intrinsic and extrinsic coagulation pathways. The effect of RBC MPs on intrinsic coagulation pathways seems complicated. Although aPTT exhibited dose dependency for isolated RBC MPs concentrations of 1000 to 20,000 per microliter, there was no significant difference between the pre-filtration and post-filtration of RBC supernatants. Since the measurement of the clotting time is too crude to indicate blood coagulability, an automated thrombogram assay which was based on detecting thrombin generation was used in this study. Thrombograms are sensitive to both low and high reactivity of the clotting system and to the influence of all kinds of antithrombotic drugs. According to the result of this study, the stored RBC supernatant may contain 10,000–20,000 RBC MPs/μL after 35 days of storage. In a patient transfused with one to four units of RBCs, the RBC MP level may increase to approximately 600–5000/μL, according to calculations based on 4200–4800 mL of circulating blood volume. Therefore, we assessed the promotion of thrombin generation at a RBC MP concentration range of 500–20,000/μL. The generation of thrombin increased in a dose-dependent manner only between RBC MP concentrations of 500 to 5000/μL with no increase being observed at higher RBC MP concentrations. This finding may explain why additional infusions of high levels of MPs from stored RBCs did not augment thrombin generation in human volunteers with endotoxemia according to a work by Peters et al.32 Removing MPs from the RBC supernatant decreased the RBC MP level to less than 100/μL and no thrombin generation could be detected. This suggests that thrombograms are sensitive enough to determine the thrombin generation directly and estimate the procoagulant potential of MPs.
Previous studies have reported that the transfusion of stored platelet or red cell supernatant to LPS-treated or untreated mice caused inflammation or coagulopathy.33-35 The incubation of RBC MPs or stored RBC supernatant with monocytes or whole blood resulted in the release or expression of various cytokines, such as IL-6, IL-1β, IL-8, CXCL-8 and TF. 14,36 In this study, results of the activation of adhesive molecules and release of inflammatory cytokines from cultured human endothelial cells were consistent with the results previously obtained from mouse cells. 12 Straat et al. reported that MPs induce a host response in a dose-dependent manner, which was also affected by the blood storage time. 37 In accordance with their research, we have previously reported that isolated RBC MPs from stored RBC supernatant primed the fMLP-activated PMN respiratory burst in a dose-dependent manner.24 The RBC MP count in this work also increased significantly during storage. The supernatant from RBCs stored for 28 to 35 days primed the fMLP-activated PMN respiratory burst effectively which very likely resulted in an increase in MPs. This result suggests that MPs from RBC supernatant is an essential factor for proinflammatory potential. However, this result differed from the conclusions of Kent et al. who reported that most priming activity from stored leukocyte-reduced RBCs is in MP-poor supernatants.9 This discrepancy may have been caused by a lower MP count in the supernatant prepared with greater centrifugation (12,500 × g for six minutes) for RBC supernatants in their experiments.
According to the two-event model of TRALI, the first event is that the patient's clinical condition causes activation of the vascular endothelium and results in PMN adhesion and activation of the vascular endothelium; the second event is caused by the transfusion to the patient of various biological response modifiers with therapeutic blood components, which causes endothelial cell damage.30 Biological response modifiers may also mediate TRALI via PMNs or macrophages.10,11 RBC MPs in stored RBCs may be more related to the onset of TRALI than other immune and non-immune factors.38 Our in vitro model of TRALI indicated that ICAM-1 expression was induced in pulmonary endothelial cells by treatment with LPS under conditions in which PMNs were allowed to adhere and activate as a first event. HMVECs were ultimately damaged by the MP-primed PMN respiratory burst as a second event, resulting in an increase of vascular and endothelial permeability, which is an important characteristic of TRALI. In this experiment, PMN was primed by isolated MPs instead of the RBC supernatant. Although the pathological mechanism of TRALI needs to be further elucidated, it was confirmed that MPs may play an important role in the development of TRALI.
ConclusionMPs in stored RBC concentrates may have an important contribution not only to systemic coagulation but also to inflammation and exert multiple effects on TRALI development. Fresh or washed RBCs are recommended for patients at risk for TRALI or thrombosis.
Data availabilityAll data are provided in full in Results. We hope readers can access the data from Results to understand the conclusions of the study.