Erythrovirus B19 (B19V), a member of Parvoviridae family, genus Erythrovirus, is a small non-enveloped DNA virus, with approximately 5000 nucleotides. There are three distinct genotypes (1, 2 and 3) with genotype 1 being the most prevalent in the world.1
B19V infection is associated with many clinical manifestations, depending on the immunological and hematological status of the patient. The virus has tropism for bone marrow erythroblasts, on which it exerts a cytotoxic effect and determines temporary suspension of erythropoiesis, leading to a transient episode of red cell aplasia.2
In sickle cell anemia (SCA) patients, B19V is known to be the etiologic agent of transient aplastic crises.3 Many other complications may be associated with B19V infection, such as acute splenic sequestration4,5 and acute chest syndrome.6
The diagnosis of B19V infection can be achieved by detecting anti-B19V antibodies or by molecular biology techniques that allow the identification of the viral DNA using direct hybridization or polymerase chain reaction (PCR), or even by direct identification of the virus by electron microscopy.7
The B19V seroprevalence increases with age and can vary from 2 to 15% in under five-year-old children, 15 to 60% for individuals aged six to 19 years, between 30 and 60% in adults, and up to 85% in the elderly population,8 both in developed and developing countries.9
In a study of 278 children with sickle cell disease (SS or Sβ0-thalassemia, median age 5.8 years; range: 0.9–12.3 years), it has been shown that past or recent viral infection occurred in 29.5% (95% confidence interval: 24.1–34.9%).5 This report describes the clinical course and the laboratory tests of two siblings selected to participate in that cohort.
Case reportsA 10-year-old male (LLS) with homozygous SS, had been regularly followed up in the outpatient clinic of the Blood Center in Belo Horizonte since the diagnosis of SCA by the Newborn Screening Program of Minas Gerais, Brazil. He was admitted to the emergency room of the João Paulo II Children's Hospital in Belo Horizonte with a history of back pain, headache and fever of up to 38.7°C for two days prior to admission. Physical examination revealed a heart rate of 90bpm and liver 3cm below the costal margin, spleen not palpable and anicteric. On the day of admission, he had two episodes of vomiting, and slurry evacuation. Respiratory symptoms were absent. Low back pain subsided the same day and the headache became intermittent. The vomiting and diarrhea receded the following day. Ampicillin was initiated upon admission but was discontinued the next day, since there was no fever or other symptoms of bacterial infection, and radiographic evaluation showed that chest and face were normal. Blood counts are shown in Table 1.
Blood counts during transient aplastic crises in two siblings with sickle cell anemia.
| Sibling 1 | Sibling 2 | |
|---|---|---|
| Age at aplastic crisis (years) | 10 | 12 |
| Gender | M | M |
| Genotype | SS | SS |
| At admission (Day 1) | ||
| Hemoglobin (g/dL) | 5.4 | 3.4 |
| Hematocrit (%) | 16 | 11.3 |
| Leukocytes (×109/L) | 3.5 | 9.2 |
| Neutrophils (%) | 86 | 54 |
| Reticulocytes (%) | Not done* | Not done* |
| Platelets (×109/L) | “Normal” | 301 |
| Day 2 | ||
| Hemoglobin (g/dL) | 6.0 | 4.5 |
| Hematocrit (%) | 19.9 | 14 |
| Leukocytes (×109/L) | 5.2 | 6.6 |
| Neutrophils (%) | 58 | 38 |
| Reticulocytes (%) | 0.3 | 0.1 |
| Platelets (×109/L) | 240 | 282 |
| Day 4 | ||
| Hemoglobin (g/dL) | 5.6 | Not done |
| Hematocrit (%) | 17.4 | Not done |
| Leukocytes (×109/L) | 7.2 | Not done |
| Neutrophils (%) | 16 | Not done |
| Red cell transfusion | 10mL/kg (Days 2 and 5) | 10mL/kg (Days 1 and 3) |
| Hospital discharge | Day 6 | Day 4 |
Before the hospital admission, this patient had been randomly selected for a research study aiming to investigate B19V infection in children with SCA, as previously mentioned. His serum sample had been drawn 16 months before the transient bone marrow hypoplasia event. In that sample no anti-B19V antibodies (IgG or IgM class – Biotrin, Ireland) had been detected, nor had viral DNA by quantitative PCR (in-house test). One year after the episode of erythroid hypoplasia a new serum sample of the patient was drawn, as recommended by the study. This sample was positive for anti-B19V IgG antibodies and negative for IgM antibodies and viral DNA.
A 12-year-old male (ALS), sibling of “Case 1”, was also regularly followed up in the outpatient clinic at Fundação Hemominas since the diagnosis of SCA in the newborn period.
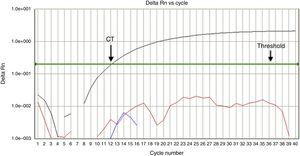
The patient was admitted to the same hospital 13 days after his brother. Before hospital admission, his symptoms had been headache and runny nose without fever for one week. They subsided spontaneously, but after three days, he presented a headache associated with vomiting and a fever peak of 38.7°C. Ibuprofen was prescribed at a government health clinic and the symptoms receded. One day prior to hospital admission, he again had headaches associated with pain in the cervical spine and vomiting. His mother reported that the degree of her son's pallor had clearly increased. At admission, he was slightly dehydrated, severely pale and mildly jaundiced. Heart and respiratory rates were 110 bpm and 26 breaths per minute, respectively; blood pressure was 110/70mmHg, his liver was 7cm from the costal margin, and spleen was not palpable. Blood counts are also shown in Table 1. B19V DNA was detected by real time PCR, and typed as genotype 1 (Figure 1).
In-house real time polymerase chain reaction assay using specific hydrolysis probes to detect erythrovirus B19 genotypes 1, 2, and 3. Positive amplification for genotype 1 (black line) was detected in Case 2. The horizontal green line represents the threshold for positivity. Intersection of this line with the black line (arrow) indicates the cycle amplification threshold (Ct) value. The early Ct=12 points for a high virus load. Fluorescent signals below the threshold indicate negative results for genotypes 2 and 3.
B19V infection causes significant morbidity in children with SCA. Although studies have been reported on the subject, there are still limited data on the epidemiology of this infection, as well as the complications associated with it.
Intrafamilial transmission of B19V infection is considered an important event for viral spread. It has been demonstrated that the single risk factor for B19V seroconversion in a child was the presence of siblings with a recent B19V infection (odds ratio: 2.97; 95% confidence interval: 1.29–6.81).2 The rate of secondary infection in families with two or more children with sickle cell disease was 56.3%.
It is known that the vast majority of children with SCA who have serologic evidence of previous B19V infection had not developed symptomatic aplastic crisis,10 as was also demonstrated by our recent cohort study.5 Different degrees of baseline hemoglobin concentration, virus load, virus genotypes or other unknown factors could explain this observation, although genotype and virus load were the same in both children during a nosocomial B19V outbreak, one child with very severe manifestations and the other with an asymptomatic course.11 It is interesting to note that the diagnosis of acute transient aplastic crisis in children with SCA who are being treated with hydroxyurea is not different to those who are not being treated with hydroxyurea. The clinical course was very similar, relapsing or chronic B19V infection was not observed, and the production of B19V-specific immunoglobulins was apparently normal.12
In conclusion, our report suggests that host immunologic background may play a significant role in the pathogenesis and clinical course of aplastic crises secondary to B19V infection, as both brothers showed life-threatening clinical manifestations. Since serological and molecular tests are not always available, the reticulocyte count is essential when transient bone marrow hypoplasia caused by B19V is suspected, so that proper supportive care can be immediately started.
Conflicts of interestThe authors declare no conflicts of interest.
Ethical approvalThe study was approved by the Human Ethics Committee on Research of Fundação Hemominas and Universidade Federal de Minas Gerais. It was conducted in accordance with the Helsinki Declaration as revised in 2008. Children and their parents signed an informed consent form.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq), Núcleo de Ações e Pesquisa em Apoio Diagnóstico, Brazil (Nupad), Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil (Fapemig).