With 99% of the cases in Brazil, malaria is endemic in the Amazon region. Transfusion-transmitted malaria, an important risk in endemic areas, has been reported. The aim of this study was to describe the epidemiological profile of blood donor candidates at the Fundação de Hematologia e Hemoterapia do Amazonas and evaluate the efficacy of rapid diagnostic tests used for malaria screening of blood donors within endemic regions.
MethodsBetween May 2008 and May 2009, 407 blood donor candidates were selected and grouped based on the Malaria Annual Parasite Index of the geographic area in which they originated: Group 1 (eligible donors – n=265) originated from areas of low to medium risk of exposure to malaria and Group 2 (ineligible donors – n=142) originated from high-risk areas. All samples were concurrently screened using two immunochromatic antigen-based rapid tests and by the thick smear test.
ResultsAll samples were negative by all three methods. The demographic profile indicated that the majority of participants were male, ages ranged from 18 to 39 years and less than half the candidates had only elementary schooling. Two issues need to be addressed: one is the ineligibility of donors and its impact on blood donor centers as, in this study, 22.7% of the donors were considered ineligible. The other is the limited sensitivity of the parasitological tests used, allowing a risk of false-negative results.
ConclusionNew methods are needed to ensure transfusion safety without rejecting potential donors, which would ensure safe transfusion without harming the blood supply.
Malaria is an acute infectious febrile disease caused by protozoan parasites from the genus Plasmodium.1 Malaria remains a significant public health problem around the world, resulting in more than one million deaths per year.2,3 In Brazil, the infection is primarily caused by Plasmodium vivax and Plasmodium falciparum; Plasmodium malariae transmission also occurs but it is the least frequently diagnosed.1,2 The Amazon region is the country's endemic area accounting for 99% of the cases diagnosed annually.4 Within the Brazilian Amazon Region, the distribution of cases is heterogeneous with risk of disease transmission being based on the Annual Parasite Index (API), calculated as the number of cases per 1000 inhabitants. High risk transmission areas are those with an API ≥50 cases/1000 inhabitants, medium risk transmission areas have between 10 and 49 cases/1000 inhabitants and low risk transmission areas are those with an API<10 cases/1000 inhabitants.4
Natural transmission occurs by the bite of an infected Anopheles sp.5 According to the Brazilian Ministry of Health there are also possibilities of neonatal infection and accidents with contaminated needles. Furthermore, transfusion-related transmission is an important risk factor in endemic areas.6 Cases of malaria caused by blood transfusion can occur when the donor has asymptomatic malaria or during the incubation period when the disease is not diagnosed by clinical and epidemiological screening.7
Post-transfusion malaria represents a challenge to hematology and hemotherapy centers located in the Amazon Region as, according to Brazilian regulations, donors originating from endemic areas are classified as high risk for malaria transmission and must be excluded. However, donors from areas classified as medium to low risk of transmission are allowed to donate blood, as long as parasitological tests have been performed.8,9 The thick blood smear is considered the gold standard to diagnose malaria; the test is based on microscopic observation of the parasite. However, this method is limited by its sensitivity and the need of well trained technicians and specialized materials.10 Even though the thick smear is the most utilized method to diagnose malaria, it cannot be performed as a high throughput test due to the impact on the flow of patient screening in hematology and hemotherapy centers that need to process many patients per day; it is thus unviable in endemic areas.10
The need to speed-up the screening and diagnostic processes has resulted in the development of new technologies to diagnose malaria including the immunochromatic antigen-based rapid tests, or simply rapid tests. These tests are based on the detection of monoclonal or polyclonal antibodies targeting specific Plasmodium antigens on a nitrocellulose strip.11 Previous studies have demonstrated that these tests usually present low sensitivity when patients are asymptomatic and with low parasite levels, posing a risk if administered in blood donor screening.12
The control of malaria in endemic regions and transfusion safety are important aspects to be considered. The real incidence of transfusion-related malaria is unknown but can be a contributing factor in the maintenance of disease transmission, especially in areas where proper physical, epidemiological and laboratorial screenings are not performed effectively. Because of the need to evaluate the malaria screening process of blood donors, a study was conducted to define the profile of those seeking the HEMOAM Foundation and to evaluate the performance of immunochromatic antigen-based rapid tests as a diagnostic tool.
MethodsA cross-sectional descriptive study was performed in blood donors with a history of exposure to malaria and the performance of two immunochromatic antigen-based rapid tests were evaluated in respect to screening potential donors in HEMOAM. Convenience sampling was employed to include potential donors that during the clinical screening and interview presented a history of possible exposure to malaria. The study enrolled 407 blood donor candidates between May 2008 and May 2009. The individuals were divided in two groups according to their level of risk (eligible and ineligible to donate blood) based on the API established by the Fundação de Vigilância em Saúde do Amazonas (FVS-AM). Group 1 (eligible) consisted of potential donors originating from areas of low to medium risk of exposure and Group 2 (ineligible) comprised of potential donors originating from high-risk areas. Donor candidates that were ineligible due to reasons other than risk of malaria were not included in this study. During the screening process at HEMOAM, potential donor candidates were approached and invited to participate in the study. Upon acceptance, all signed informed consent forms. This research was approved by the Ethics Committee of HEMOAM (#154985).
Subsequently, a questionnaire was completed on the demographic (gender, age), socioeconomic (geographic origin and education level) and epidemiological (history of previous malaria infection, visits to areas with risk of malaria transmission and place of residence) aspects of participants.
Eligible donors were sent to the blood collection room where a 5mL whole blood sample was collected in a labeled Vacutainer® blood collection tube containing ethylenediaminetetraacetic acid (EDTA: BD, USA). Ineligible donors were taken to the clinical analysis laboratory to undergo blood collection using the same procedure. At the time of blood collection, two thick smear slides were prepared using peripheral capillary blood collected from a finger prick according to World Health Organization (WHO) guidelines and evaluated by two experienced technicians from the District III Laboratory for Endemic Disease Control of FVS-AM. Additional malaria testing was performed using two immunochromatic antigen-based rapid tests: OptiMal® (DiaMed, Switzerland), a routine test at FHEMOAM, and SD Bioline Malaria Antigen® (Standard Diagnostics, INC, Korea). These tests detect the presence of Plasmodium lactate dehydrogenase (PLDH) using monoclonal and polyclonal antibodies, respectively. All essays were performed following the specifications of the manufacturers. Statistical analysis utilized the EpiInfo™ (version 3.5) computer program; the Pearson chi-square test was used to compare categorical data and Student's t-test was used to compare means. The level of significance was set at 5% (p-value<0.05).
ResultsDuring the study period, 44,117 candidates went to HEMOAM to donate blood. After clinical and epidemiological screening, 33,317 were considered eligible for donation and 10,800 were considered ineligible for differing reasons. Of 667 eligible donors, 265 agreed to participate in the study (Group 1). Of the ineligible donors, 2455 (22.7%) were not allowed to donate because they originated from high-risk areas for malaria transmission based on the API, and 142 consented to participate in this study (Group 2). Thus a total of 407 individuals were enrolled.
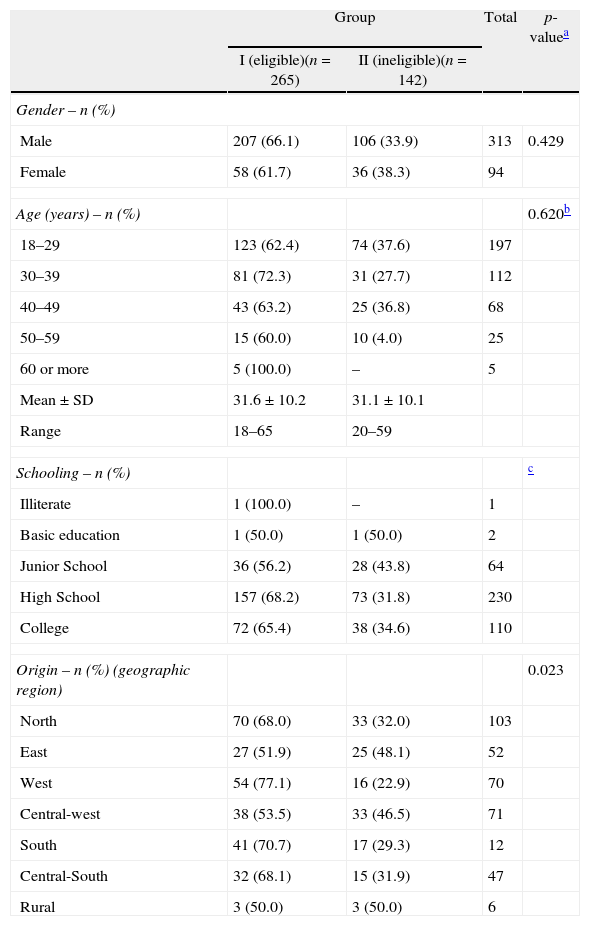
All samples were evaluated using the two immunochromatic antigen-based rapid tests and the thick smear test; results were negative in all three tests. Profile analysis of the donor candidates by groups (Table 1) showed that the only significant difference between the two groups (p-value=0.023) occurred when the residence within and around the Municipal of Manaus was considered; 68% (70/103) of candidates originating from the northern region of Manaus were in Group 1 and 32% (33/103) in Group 2. Table 1 also shows a prevalence of men, and candidates between the ages of 18 and 29 in both groups. Additionally, the majority of candidates had completed high school or college level education in both groups.
The sociodemographic profile of blood donor candidates according to the risk of exposure to malaria.
| Group | Total | p-valuea | ||
| I (eligible)(n=265) | II (ineligible)(n=142) | |||
| Gender – n (%) | ||||
| Male | 207 (66.1) | 106 (33.9) | 313 | 0.429 |
| Female | 58 (61.7) | 36 (38.3) | 94 | |
| Age (years) – n (%) | 0.620b | |||
| 18–29 | 123 (62.4) | 74 (37.6) | 197 | |
| 30–39 | 81 (72.3) | 31 (27.7) | 112 | |
| 40–49 | 43 (63.2) | 25 (36.8) | 68 | |
| 50–59 | 15 (60.0) | 10 (4.0) | 25 | |
| 60 or more | 5 (100.0) | – | 5 | |
| Mean±SD | 31.6±10.2 | 31.1±10.1 | ||
| Range | 18–65 | 20–59 | ||
| Schooling – n (%) | c | |||
| Illiterate | 1 (100.0) | – | 1 | |
| Basic education | 1 (50.0) | 1 (50.0) | 2 | |
| Junior School | 36 (56.2) | 28 (43.8) | 64 | |
| High School | 157 (68.2) | 73 (31.8) | 230 | |
| College | 72 (65.4) | 38 (34.6) | 110 | |
| Origin – n (%) (geographic region) | 0.023 | |||
| North | 70 (68.0) | 33 (32.0) | 103 | |
| East | 27 (51.9) | 25 (48.1) | 52 | |
| West | 54 (77.1) | 16 (22.9) | 70 | |
| Central-west | 38 (53.5) | 33 (46.5) | 71 | |
| South | 41 (70.7) | 17 (29.3) | 12 | |
| Central-South | 32 (68.1) | 15 (31.9) | 47 | |
| Rural | 3 (50.0) | 3 (50.0) | 6 | |
SD: standard deviation.
#: Grades 1-9 as established by the Brazilian Department of Education. &: High School as established by the Brazilian Department of Education.
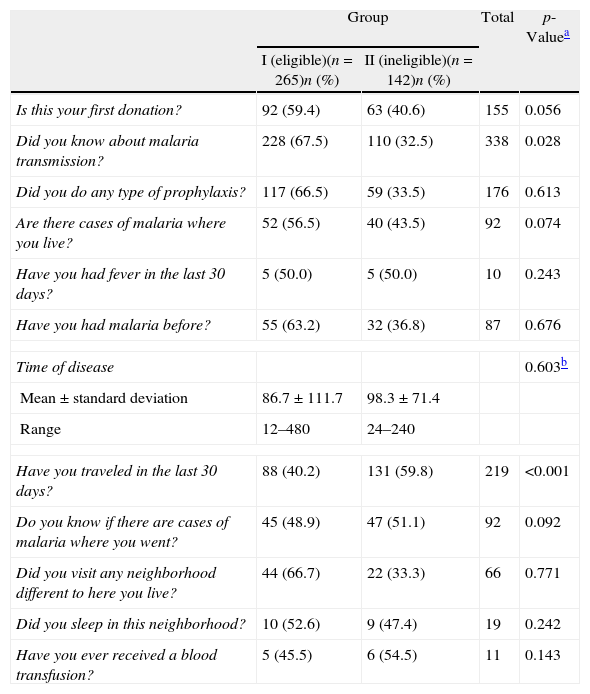
Candidates were also asked about their blood donation experience and travel history within and outside of Manaus (Table 2). When asked if they were donating blood for the first time, 155 out of 407 participants answered “yes”; with 59.3% (92/155) of the eligible and 40.7% (63/155) of the ineligible donors, there was a significant difference between the two groups (p-value=0.056). reported by 87 of the 407 candidates, 63.2% (55/87) were from the eligible group and 36.8% (32/87) from the ineligible group (non-significant difference); however, all of the ineligible candidates reported traveling outside of Manaus within the 30 days preceding the interview (p-value<0.001).
Clinical and epidemiological data of blood donor candidates in Group I (eligible) and Group II (ineligible).
| Group | Total | p-Valuea | ||
| I (eligible)(n=265)n (%) | II (ineligible)(n=142)n (%) | |||
| Is this your first donation? | 92 (59.4) | 63 (40.6) | 155 | 0.056 |
| Did you know about malaria transmission? | 228 (67.5) | 110 (32.5) | 338 | 0.028 |
| Did you do any type of prophylaxis? | 117 (66.5) | 59 (33.5) | 176 | 0.613 |
| Are there cases of malaria where you live? | 52 (56.5) | 40 (43.5) | 92 | 0.074 |
| Have you had fever in the last 30 days? | 5 (50.0) | 5 (50.0) | 10 | 0.243 |
| Have you had malaria before? | 55 (63.2) | 32 (36.8) | 87 | 0.676 |
| Time of disease | 0.603b | |||
| Mean±standard deviation | 86.7±111.7 | 98.3±71.4 | ||
| Range | 12–480 | 24–240 | ||
| Have you traveled in the last 30 days? | 88 (40.2) | 131 (59.8) | 219 | <0.001 |
| Do you know if there are cases of malaria where you went? | 45 (48.9) | 47 (51.1) | 92 | 0.092 |
| Did you visit any neighborhood different to here you live? | 44 (66.7) | 22 (33.3) | 66 | 0.771 |
| Did you sleep in this neighborhood? | 10 (52.6) | 9 (47.4) | 19 | 0.242 |
| Have you ever received a blood transfusion? | 5 (45.5) | 6 (54.5) | 11 | 0.143 |
Malaria is a reason for a continuous state of alert during blood donor screening, especially in endemic areas. Blood donation eligibility in Brazil takes into consideration the risk of exposure to malaria as measured by the API of the geographic region where blood donor candidates originate. In this study we evaluated a sample of potential blood donors, clinical and epidemiological data were collected and individuals were tested for malaria using two immunochromatic antigen-based rapid tests and the gold standard to diagnose malaria, the thick smear test. In this study there were no positive results for malaria among both eligible and ineligible candidates.
Even though the thick blood smear is the gold standard, it requires time and expertise, especially when the parasite blood levels are low.11 Operational issues became an obstacle in the use of microscopy in the routine screening of potential donors in blood banks serving large populations and with a high throughput of samples.10,11 In 2010, Ali et al. in a study carried out in Pakistan, tested 1558 blood donors using microscopy to diagnose malaria, and found nine (0.577%) positive tests.13 However, this is not a viable approach for medium and large donation centers.
The immunochromatic antigen-based rapid malaria tests have been developed because of the need of fast and accurate results.10 Some of these methods utilize monoclonal and polyclonal antibodies directed against the P. falciparum histidine-rich protein 2 (PfHRP-2) and against the Plasmodium lactate dehydrogenase (PLDH) of four Plasmodium species.11 These are rapid and efficient tests but lack the ability to diagnose mixed infections and present limited sensitivity when parasite levels are low.10
In 2007, Ratsimbasoa et al. compared the rapid tests, SD Malaria, OptiMAL and CareStart, against the thick blood smear in samples from an endemic area in Madagascar. The sensitivity for P. falciparum was 89.4%, 92.6% and 97%, respectively. All three tests were less sensitive in non-endemic areas for P. falciparum and when parasite levels were less than 500 parasites/μL of blood.14 Contreras et al. emphasize that, despite uncommon, malaria cases resulting from transfusions are usually lethal, and, in endemic areas, transfusion-related malaria is indistinguishable from natural malaria infection. Individuals presenting asymptomatic infection by Plasmodium parasites represent a risk for transfusion-related transmission; these are usually cases with low parasite levels that are undetected in the screening process using less sensitive laboratory tests.7,15
Many people refused to participate due to the inconvenience of having to collect blood to test for malaria; the finger prick is considered painful, and even though candidates voluntarily offered to donate blood, they were unwilling to participate in this study.
In this study, there was a prevalence of men in both groups, a characteristic observed in many blood banks in Brazil.13 The mean age of candidates was 31 years, ranging from 18 to 65 in Group 1 and from 20 to 59 in Group 2; 48% were between 18 and 29, an age that many individuals participate in outdoor activities common to the Amazon region which increase the risk of exposure to the disease. These activities include mandatory military service which often entails staying inside the forest. Also, the younger Brazilian population is more open to new ideas and is becoming more active and involved in social programs such as blood donation.13
Education is a deciding factor in enrolling and maintaining donors, as well as establishing the role of a citizen and a socially responsible individual.16 In this study 56.5% had high school diplomas [157/230 (68.2%) in Group 1 and 73/230 (31.8%) in Group 2] and another 27% had college education [72/110 (65.4%) in Group 1 and 38/110 (34.6%) in Group 2]. We speculate that this population has been exposed to a level of knowledge that breaks the taboos associated with blood donation often found in developing countries,23,24 and we hope that as education improves in Brazil more people will volunteer to donate blood.
The incidence of malaria in the Amazon Region has seasonal fluctuations. In this region the temperature is stable, but the rainfall and humidity vary during the year and malaria transmission follows a pattern associated with the seasons.16,17 In our study, potential donors were selected throughout the year but no positive samples were detected, we understand that the small study size and convenience sampling may have influenced this result and only a larger, more structured study would help to find the real incidence of malaria infection within potential blood donors.
The current criteria of eligibility for blood donation based on the patient's origin and visits to a high-risk area have a negative impact on the supply of blood and blood related products. In the Unites States, the ineligibility of donors related to visiting malaria endemic areas has been questioned and it has been demonstrated that the screening criteria should be reassessed due to the impact on the blood supply.18
Manaus is divided into six city zones; North, East, West, Central-west, South, Central-south and the rural area. In this study, there was a statistically significant difference in the geographic region of the houses of potential donors (p-value<0.001), with 68% (70/103) of eligible and 32% (33/103) of ineligible living in the North of the city. This data correlates with the origin of patients with malaria diagnosed and notified by the Foundation of Tropical Medicine from the Amazonas where the majority comes from West, East, North and Rural areas of Manaus,19 where there is a high concentration of population settlements and disorderly occupations of land.
We found that 56.5% (230/407) of the study participants visited high risk areas for the transmission of malaria within 30 days prior to volunteering to donate blood; 61.7% (142/230) of these were in Group 2; individuals who visit these areas cannot donate blood. We also discovered that three candidates had a history of malaria within 12 months of offering to donate blood and that five had fever within 30 days prior to their visit to donate blood. These individuals were considered fit to donate by routine screeners when they should have been considered ineligible. This demonstrates that the screening process is not error-proof, and even with a negative result in all three tests, this fact supports the need for a better, more rigorous screening process and also the need of tests sensitive enough to detect low parasite levels.
Many previous reports have emphasized the use of molecular techniques, especially DNA amplification using polymerase chain reaction (PCR), which has higher sensitivity than other methods, including the thick blood smear, the current gold standard.20,21 In 2006, Torres et al. diagnosed, using PCR, one patient with P. vivax DNA among 11 cases who presented symptoms for the disease but had negative parasite exams. Fugikaha et al. evaluated the frequency of blood donors infected with malaria in four blood banks in areas within the Brazilian Amazon. Of the 400 donors submitted to nested-PCR evaluations, nine were positive for malaria, revealing high positivity, varying from 1 to 3%, for blood donors in the four centers.22
ConclusionIn this study, all samples tested using the rapid immunochromatic antigen-based malaria tests, OptiMal® and SD Bioline Malaria Antigen® and the thick blood smear, the gold standard to diagnose malaria, were negative and so no significant difference in sensitivity or specificity was found.
The majority of the donor candidates were male, young adults, had either high school or college education and originated from the northern region of Manaus.
More research including the use of molecular biology techniques are needed to evaluate the risk of transfusion-related malaria transmission, with the ultimate goal of reviewing the guidelines of the screening process in hematology and hemotherapy services in endemic areas.
Conflicts of interestThe authors declare no conflicts of interest.
Financial support was provided by the Fundação de Amparo à Pesquisa do Estado do Amazonas (#PIPT 009/2007). The authors extend their special thanks to the technical collaborators Alcineide Pereira Vasconcelos, Ana Ruth Arcanjo, Edson da Fonseca Lira, Felicien Vasquez,Fernanda Braga, Shirley Araniva, Sthefanny Azevedo; to the Fundação de Vigilância em Saúde do Amazonas, to the Laboratório de Controle de doenças Infeciosas distrital III, to the Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas; to SD Incorporation for the donation of the SD malaria® detection kits and to Dr. Patricia D. Santos-Ciminera, Adjunct Professor of Biology, Stevenson University, MD, USA for the critical review of this manuscript and assistance with translation to English.