Oral manifestations may be the first signs of hematologic diseases, and may occur due to the disease itself or to treatment.
ObjectiveTo evaluate the frequency and types of oral conditions presented by patients on a hematology ward.
MethodsData were collected by oral examinations during weekly visits to a hematology ward. Six trained dentists performed the oral assessment based on the principles of oral semiology. All patients who accepted to be examined were included in the study. Patients who were unavailable or unable to have oral examinations were excluded. Data were recorded on protocol forms and in the electronic records of the institution. A descriptive analysis was performed.
ResultsSeventy-nine patients were included in the analysis; 50.6% were female and the mean age was 41.49 years. The most common reasons for hospitalization were chemotherapy and complications (81%), relapse (13.9%) and pre-transplant preparation (5%). The most frequent underlying diseases were multiple myeloma (17.7%), acute myeloid leukemia (15.4%) and acute lymphocytic leukemia (11.5%). Oral conditions were found in 36 (45.6%) patients, some of whom presented more than one condition. The most common oral conditions were dry lips (12.6%), mucositis (10.1%), petechiae (8.9%) and candidiasis (7.6%). Of the detected oral conditions, 56.9% were related to the underlying disease or chemotherapy and 20.2% were not related to the disease.
ConclusionThis study shows the types and frequency of oral conditions observed in hematological inpatients. Awareness of these conditions is important for prevention and planning the care of patients with hematological diseases.
Oral lesions may be the first signs of hematologic diseases. Dentists may be the first healthcare professionals to detect oral signs related to a systemic condition and thus contribute to the early diagnosis of the disease.1 Oral health is important for systemic balance and oral conditions aggravate the progression of some diseases2; some patients with hematologic diseases evolve with complications due to oral infections, which result in longer hospital stay and increased costs.2 The oral manifestations of patients with hematologic diseases can occur due to the disease itself or as a consequence of treatment.3
Anemia, clotting disorders or neoplasms are some of the signs of hematologic diseases.1,4 Oral petechiae or ecchymosis, and spontaneous gingival bleeding without a local cause, may be an oral manifestation of a hematologic problem.5 Moreover, paleness of the oral mucosa and ulcers are associated with anemia and gingival overgrowth and persistent infections may be signs of leukemia.4
The treatment of hematologic diseases includes chemotherapy, radiotherapy, and hematopoietic stem cell transplantation (HSCT).4 Malignant cells are the target of antineoplastic drugs, but the oral epithelium and other cells with high mitotic rates are usually affected by the treatment. Chemotherapy and the conditioning regimen for HSCT have many adverse effects on the oral tissues depending on the type and dosage of medications. Oral mucositis, a common side effect of chemotherapy, causes severe pain, which is only relieved by opioids.6,7 Mucositis may appear as a generalized erythema and evolve into painful pseudomembranous ulcers.8 Neutropenia and thrombocytopenia are also common adverse effects of treatment, leaving patients more susceptible to oral bleeding, infections, and ulcerations.9 Oral mucosa pigmentation can also occur as an adverse effect of treatment.9
Complications of HSCT can affect most of the organs with oral tissues being affected in the early and late periods after HSCT.10 Oral chronic graft-versus-host disease is a late complication of HSCT that affects nearly 80% of the patients.11 Salivary flow rates are often reduced due to chemotherapy or to chronic graft-versus-host disease and may cause many oral complications, such as varying degrees of discomfort, dysphagia, dysphasia, dysgeusia, halitosis, and infections such as dental decay, periodontitis and candidiasis.12
The most common oral complications in the treatment of hematologic diseases are mucositis, bleeding, hyposalivation, fungal or viral infections and aggravation of odontogenic infections.5,13,14 The aim of this study was to evaluate the frequency and types of oral conditions found in patients on a hematology ward during routine dental visits.
MethodsThis is a retrospective study with data collected from the patients of the Hematology ward of the Clementino Fraga Filho University Hospital (HUCFF) of the Universidade Federal do Rio de Janeiro (UFRJ). Data used in this study were collected from routine weekly oral examinations carried out from July 2007 to September 2009 in the patients on the hematology ward. All patients willing to be examined were included in the study, even if they did not present any complaints. The patients that were unable to have oral examinations for any reason that impaired the examination such as intubation or sedation, were excluded from the study. The need for signed informed consent was waived by the institution review board as data were collected from patient records.
Clinical and demographic data were collected from electronic medical records and oral data were collected from the routine exams. Data collected from oral exams were recorded on a standard form, designed specifically for the visits to the wards, and included the patient identification, demographic information, underlying disease, reason for hospitalization and medications including chemotherapy.
A team of four stomatologists and two general dentists were involved in the oral examination of the patients. Training was provided for the two general dentists by exhibiting images of normal mucosa and its variations with clinical discussion on the differential diagnoses of oral lesions.
The intraoral examinations were performed at the bedside with the aid of a frontal light emitting diode light, while respecting biosafety guidelines, and principles of oral semiology.15 The steps to evaluate the oral mucosa were standardized in the following order: vermillion lip border, labial mucosa, buccal mucosa, gingiva, tongue (dorsum, lateral and ventral surfaces), hard and soft palate, and oropharynx, on both sides of the mouth.
The diagnoses of the oral findings were based on clinical examinations and laboratory exams, when required. If oral changes were detected, the medical team was notified and the patient was treated by a stomatologist of the Oral Health Program team of the same hospital.
Oral conditions detected during examinations were grouped into conditions related to the underlying disease or to its treatment and those that were not related. The following conditions were considered changes related to the underlying disease or to its treatment: mucositis, bleeding disorders, dry lips or mucosa, fungal or viral infections, and aggravation of odontogenic infections.
A descriptive analysis of the data was performed. The collected data were stored and analyzed using the Statistical Package for the Social Sciences (SPSS) 13.0 program for Windows (IBM SPSS©, Chicago, the United States).
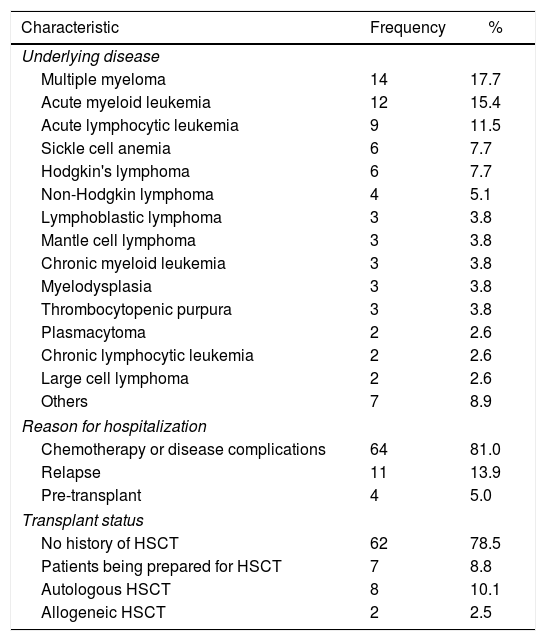
ResultsData on 138 oral exams performed in 79 patients on the hematology ward were included in the analysis. The mean age of the patients was 41.5 years old (range: 14–78 years old) and 50.6% were male. The clinical data of the study population are shown in Table 1.
Clinical characteristics of the 79 patients.
| Characteristic | Frequency | % |
|---|---|---|
| Underlying disease | ||
| Multiple myeloma | 14 | 17.7 |
| Acute myeloid leukemia | 12 | 15.4 |
| Acute lymphocytic leukemia | 9 | 11.5 |
| Sickle cell anemia | 6 | 7.7 |
| Hodgkin's lymphoma | 6 | 7.7 |
| Non-Hodgkin lymphoma | 4 | 5.1 |
| Lymphoblastic lymphoma | 3 | 3.8 |
| Mantle cell lymphoma | 3 | 3.8 |
| Chronic myeloid leukemia | 3 | 3.8 |
| Myelodysplasia | 3 | 3.8 |
| Thrombocytopenic purpura | 3 | 3.8 |
| Plasmacytoma | 2 | 2.6 |
| Chronic lymphocytic leukemia | 2 | 2.6 |
| Large cell lymphoma | 2 | 2.6 |
| Others | 7 | 8.9 |
| Reason for hospitalization | ||
| Chemotherapy or disease complications | 64 | 81.0 |
| Relapse | 11 | 13.9 |
| Pre-transplant | 4 | 5.0 |
| Transplant status | ||
| No history of HSCT | 62 | 78.5 |
| Patients being prepared for HSCT | 7 | 8.8 |
| Autologous HSCT | 8 | 10.1 |
| Allogeneic HSCT | 2 | 2.5 |
HSCT: hematopoietic stem cell transplantation.
The chemotherapy regimens were established according to the institution protocol. The other frequently prescribed medications were antibiotics (60.7%), analgesics (45.6%) and diuretics (22.8%).
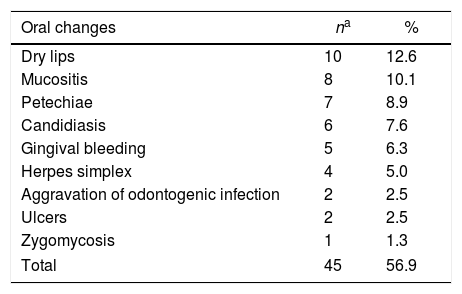
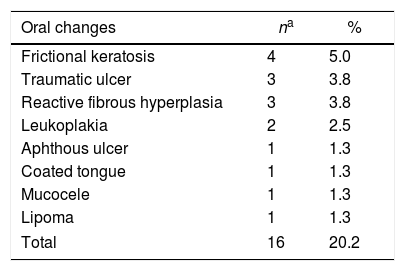
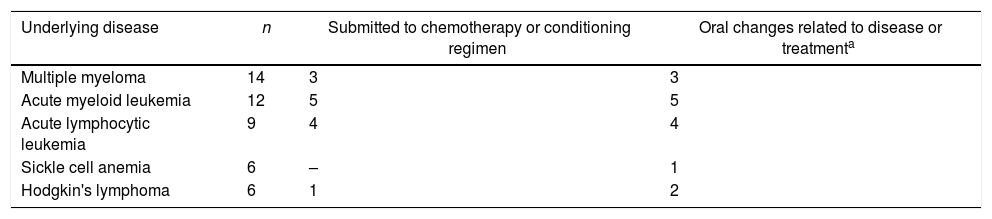
Oral findings of the patients related to the underlying disease or to its treatment are listed in Table 2 and those not related to the disease are listed in Table 3. Some of the patients presented more than one oral alteration. A total of 45 oral conditions were observed in 36 patients (45.6%). of the conditions, 45 (56.9%) were related to the underlying disease or treatment and 16 (20.2%) were not related to the disease. Table 4 shows the oral conditions according to the most frequent underlying disease. Of the patients submitted to HSCT, 20% presented oral conditions related to treatment.
Oral conditions associated to the underlying disease or chemotherapy observed in the 79 patients.
| Oral changes | na | % |
|---|---|---|
| Dry lips | 10 | 12.6 |
| Mucositis | 8 | 10.1 |
| Petechiae | 7 | 8.9 |
| Candidiasis | 6 | 7.6 |
| Gingival bleeding | 5 | 6.3 |
| Herpes simplex | 4 | 5.0 |
| Aggravation of odontogenic infection | 2 | 2.5 |
| Ulcers | 2 | 2.5 |
| Zygomycosis | 1 | 1.3 |
| Total | 45 | 56.9 |
Oral conditions not related to the underlying disease observed of 79 patients.
| Oral changes | na | % |
|---|---|---|
| Frictional keratosis | 4 | 5.0 |
| Traumatic ulcer | 3 | 3.8 |
| Reactive fibrous hyperplasia | 3 | 3.8 |
| Leukoplakia | 2 | 2.5 |
| Aphthous ulcer | 1 | 1.3 |
| Coated tongue | 1 | 1.3 |
| Mucocele | 1 | 1.3 |
| Lipoma | 1 | 1.3 |
| Total | 16 | 20.2 |
Oral conditions according to the most common underlying diseases.
| Underlying disease | n | Submitted to chemotherapy or conditioning regimen | Oral changes related to disease or treatmenta |
|---|---|---|---|
| Multiple myeloma | 14 | 3 | 3 |
| Acute myeloid leukemia | 12 | 5 | 5 |
| Acute lymphocytic leukemia | 9 | 4 | 4 |
| Sickle cell anemia | 6 | – | 1 |
| Hodgkin's lymphoma | 6 | 1 | 2 |
Almost half of the study patients (45.6%) on the hematology ward presented oral conditions. Most of these conditions (56.9%) were related to the underlying disease or to its treatment. This is in accordance with studies that reported almost 40% of oncologic patients under chemotherapy regimens presenting oral lesions.4,5,16 The frequency of these conditions varies between studies, depending on the characteristics of the study population, the chemotherapy regimen, the evaluation methods, and the patient's oral health status.6,17
Chemotherapy is a common treatment for hematologic malignancies, and often causes a variety of complications, including sepsis and death.16 In the present study, the majority of the patients (81%) on the ward were hospitalized for chemotherapy or complications related to treatment.
The oral tissues are highly sensitive to the toxic effects of antineoplastic drugs.18 This explains the fact that oral lesions are one of the most common complications of chemotherapy.5,19 The mucotoxic effects of frequently used drugs such as cytosine arabinoside, daunorubicin and methotrexate, directly affect the oral mucosa causing mucositis.18
Mucositis occurs a few days after the beginning of chemotherapy and affects approximately 30% to 40% of patients; it affects up to 90% of the patients who undergo HSCT.7,8 In the study population, only 10.1% of the patients presented mucositis. The reason for this low frequency may be the long time after HSCT, as many patients were hospitalized because of treatment complications or relapse. Other possible reasons are the diversity of the protocols and chemotherapy regimens, and factors such as age, type of the disease and duration of treatment.6
Dry lips was the most common oral condition (12.6%). On considering only those patients who underwent chemotherapy, 31.3% of the patients suffered from dry lips. Patients with dry lips often complain of xerostomia.20 Unfortunately, sialometry is not part of the routine oral exam of the patients on the ward. Xerostomia and hyposalivation are frequent side effects of chemotherapy and many other drugs.14 In fact, many xerogenic drugs, such as diuretics, antihypertensive and antihistamine drugs were administered to the patients in this study. Dry lips may also be aggravated by the use of air conditioning on hospital wards, which leads to a dry environment.
The prevalence of xerostomia varies according to the antineoplastic treatment. Xerostomia has been reported as a complication of chemotherapy in 33.3% of the cases.18 Patients who underwent HSCT had 50% to 90% reductions in the salivary flow rates and altered salivary composition.11 Analysis of the salivary glands obtained from necropsies of patients treated with different chemotherapy protocols, showed ductal enlargement of the minor salivary glands and acinar degeneration in 50% of patients.9
Thrombocytopenia is a frequent known side effect of chemotherapy. Gingival bleeding and petechiae were observed in 15.2% of the study patients. The most common underlying disease in patients with oral bleeding disorders were acute myeloid leukemia (41.7%), acute lymphocytic leukemia and mantle cell lymphoma (16.7% for both), and chemotherapy was the most frequent reason for hospitalization. None of these patients was a HSCT recipient. No epidemiological studies reporting the prevalence of oral hemorrhagic disorders were found, so frequencies could not be compared. It is important to distinguish the bleeding of hematologic disorders from bleeding associated to gingivitis, which is generally caused by poor oral hygiene. Furthermore, it is important to distinguish petechiae caused by trauma, from those related to hematologic disorders or as a side effect of chemotherapy.1,4
The oral health condition may interfere in morbidity and disease prognosis.9,17 Oral infections can be a source of bacteremia that sometimes complicates the prognosis; infections of different etiologies occurred in 16.4% of the patients in this study. Infections related to antineoplastic chemotherapy have been reported in 70% of the patients submitted to immunosuppression.13 Oral bacterial infections are not always easy to identify because patients do not present the classic signs; there are no usual signs of abscess or suppuration because leukocytes are reduced in neutropenic patients.2 Therefore, fever may be the only sign of the infection.
Of the cases, 2.5% were diagnosed as exacerbations of bacterial odontogenic infections. A detailed approach to dental exams was not the aim of the oral examinations. When these problems were identified, the patients were referred to the Oral Health Program team of the institution. The extraction or treatment of infected teeth should be planned prior to chemotherapy, since abnormal bleeding, insufficient wound healing and local infection can delay the hematologic treatment.13,15
The most frequent oral fungal infection is candidiasis. In the oral tissues, candidiasis may appear as pseudomembranous white plaques, erythematous areas, chronic atrophic white plaques, or angular cheilitis.19,21 Immunocompetent patients rarely present oral candidiasis.22 In terminal cancer patients, Candida colonization was detected in 26% of the patients, but clinical findings showed angular cheilitis in 11% and pseudomembranous candidiasis in 9%.19 In the present study, 7.6% presented oral candidiasis with all diagnoses being confirmed by culture. Each patient presenting oral candidiasis had a different systemic condition. Fungal infections occur less frequently than bacterial infections, but Candida infection is responsible for 85% of septicemia in cancer patients with a higher rate of mortality than other infections.19 Fifteen percent of HSCT recipients develop systemic fungal infections with candidiasis being the most common.18 There was also a rare case of zygomycosis of the mandible.
Viral infections by herpes simplex (HSV) and herpes zoster are not rare in myelosuppressed patients; HSV is more commonly associated in patients with neoplasms. In cases of patients submitted to HSCT, the incidence of HSV reactivation ranges from 50% to 90%.23 In the oral mucosa, HSV lesions appear as ulcerative coalescent lesions after three to four weeks of chemotherapy or HSCT.24 In this study, oral HSV infection was observed in 5% of the patients. From the four patients with HSV infection, only one had been submitted to HSCT, and this patient was hospitalized due to fever. Data from this study are in agreement with other studies; the prophylactic use of acyclovir can reduce the incidence of HSV infection in cancer patients.23
Many difficulties concern the oral care of patients prior to chemotherapy or HSCT. Professionals designated exclusively to the care of these patients are not always available. The role of the dentist is essential in a multidisciplinary team for the care of hematologic patients in order to prevent, diagnose and control preexisting problems such as tooth decay, periodontal diseases and other alterations of the oral and perioral tissues.
In the present study, 20.2% of the patients presented oral lesions that were not associated to the underlying disease or chemotherapy. The most common lesions were frictional keratosis, traumatic ulcers and reactive fibrous hyperplasia. These data are in agreement with data collected in a prevention campaign in stomatology held in the same geographic area, in which oral changes were detected in 21.8% of the general population.25
This study presents some limitations. Firstly, missing information on patients records is a common problem in retrospective studies. Moreover, bedside examinations are restricted to clinical findings, with no available imaging resources, precision periodontal instruments or any other auxiliary methods to evaluate the oral tissues. Whenever additional exams were required, the patients were referred to the Oral Health Program clinic.
The results of this study may contribute by providing data on the frequency of oral conditions. Dentists need to be included as part of the multidisciplinary team so that preventive interventions can be accomplished for the oral health of patients with hematological diseases. Future studies should consider collecting standardized detailed information on disease and transplant status, and the timeline when oral evaluations occur. Future studies should also be performed to help reduce the pain and discomfort caused by oral alterations, and to verify if dental interventions in the hematologic ward will improve the prognosis of patients.
Conflicts of interestThe authors declare no conflicts of interest.