Telomeres, the ends of linear chromosomes, shorten during mitotic cell division and erosion may be aggravated by inflammation or proliferative and oxidative stress. As the bone marrow is under hyperproliferative pressure in sickle cell disease and several tissues are submitted to chronic inflammation, this study sought to determine the telomere length of patients with sickle cell disease.
MethodsThe mean telomere length was measured in peripheral blood leukocytes by quantitative polymerase chain reaction. The age-adjusted telomere to single copy gene ratio was compared between 91 adult sickle cell disease patients and 188 controls.
ResultsSickle cell disease patients had significantly shorter telomeres than the controls (p-value<0.0001). Moreover, among sickle cell disease genotypes, Hb SS patients had significantly shorter telomeres compared to Hb SC and Hb Sβ patients (p-value<0.0001). Patients on hydroxyurea also had shorter telomeres in comparison to those off the drug (p-value=0.02). A positive correlation was observed between telomere length and hemoglobin level (r=0.3; p-value=0.004), whereas negative correlations were detected between telomere length and lymphocyte count (r=−0.3; p-value=0.005) and interleukin-8 serum levels (r=−0.4; p-value=0.02).
ConclusionsThe findings of this study indicate that telomeres are short in sickle cell disease patients and that telomere erosion directly correlates with disease genotype, inflammation markers, and the use of hydroxyurea.
Sickle cell disease (SCD) is characterized by the presence of a pathological hemoglobin, denominated hemoglobin (Hb) S. The formation of Hb S is caused by a mutation in the β-globin gene at the 17th nucleotide, in which thymine is changed to adenine, resulting in the substitution of the sixth amino acid of the β-globin chain from glutamic acid to valine. The term SCD includes several genotypes with the presence of βS allele, including the homozygous form (Hb SS or sickle cell anemia) and heterozygous forms with co-inheritance of other mutations such as βC allele (Hb SC or Hb SC disease) and the β-thalassemia allele (Hb Sβ or SβThalassemia). The clinical hallmarks of SCD are chronic intravascular hemolysis and acute vaso-occlusive events, which involve endothelial dysfunction, increased cellular adhesion, chronic inflammation, leukocytosis, coagulation activation and oxidative stress.1 Hemolysis plays a central role in the disease mechanism due to the constant release of free hemoglobin and free heme from red blood cells (RBC) into plasma, leading to nitric oxide (NO) depletion causing potent immediate systemic and vascular inflammation.2 NO depletion may lead to a highly adhesive endothelium, and platelet and coagulation activation.3 Unbound extracellular heme causes the generation of reactive oxygen species (ROS) and activation of innate immunity pathways through Toll-like receptor 4 (TLR4).4,5
Telomeres are hexameric T2AG3 tandem repeats coated by specialized proteins covering the ends of linear chromosomes.6 Telomeres confer protection against chromosome instability and activation of the DNA-damage response (DDR) machinery. Telomere shortening is a chronological marker of aging, and its accelerated shortening rate has been involved in the development of several diseases, the telomeropathies.7 In addition, cumulative events resulting from replicative stress, such as exposure to biochemical stressors, excessive oxidation, and inflammation, may cause excessive telomere erosion.8,9 Maintenance of telomere integrity requires telomerase reverse transcriptase (TERT), its RNA template (TERC), and other proteins, which compose the telomerase complex.
In view of the inflammatory and oxidative stress features observed in SCD, the telomere length was determined in SCD patients and healthy controls in order to correlate this with disease genotype, severity, and inflammation markers.
MethodsPatients and controlsThis study was performed in a cohort of adult SCD patients seen at the Outpatient Clinic of the Hematology and Hemotherapy Center, Universidade Estadual de Campinas (UNICAMP). Patients that were homozygous for Hb S (Hb SS), heterozygous with Hb C and S (Hb SC), and heterozygous with β-thalassemia and S (Hb Sβ) were included. All of the patients were in steady state therefore none had had painful crises, hospitalizations or blood transfusions during the three months preceding blood sample collection. A group of 188 age-matched healthy subjects (Hb AA) were analyzed as controls for telomere length measurement.10 An additional 70 age- and gender-matched healthy subjects (Hb AA) were evaluated for lymphocyte counts and interleukin-8 (IL-8) levels. Venous blood samples for all study analyses, including peripheral blood counts and hemolysis markers, were obtained during clinic visits. The University's Ethics Committee approved the study and all patients gave their written informed consent.
DNA extractionGenomic DNA was extracted from the buffy coat of healthy controls’ peripheral blood leukocytes up to 48h after collection, using the Gentra Purege Blood Kit (Qiagen, Maryland, USA). DNA samples were quantified, diluted to 50ng/μL and stored at −20°C. Genomic DNA from SCD patients was isolated from frozen white blood cells (WBC). Briefly, whole peripheral blood samples were washed in phosphate buffered saline (PBS) with 0.1% bovine serum albumin (BSA) up to 16h after collection and incubated on ice cold ammonia chloride (NH4Cl) for osmotic red blood cell lyses. WBCs were counted, aliquoted, and frozen at −80°C in 10% dimethyl sulfoxide (DMSO) until defrosting and DNA extraction, performed using the Gentra Purege Blood Kit (Qiagen, Maryland, USA). Genomic DNA (50ng/μL) was checked for integrity in 0.8% agarose gel at 80V for 40min. For quantitative polymerase chain reaction (qPCR), DNA dilutions of 5ng/μL were prepared and kept at 4°C up to seven days, when the subsequent dilutions of 0.2ng/μL were used for every run prepared just before experiments.
Leukocyte telomere length measurement by quantitative polymerase chain reactionThe mean leukocyte telomere length was determined by qPCR as has been described previously.11–13 All qPCR reactions were prepared on a QIAgility automated pipettor (Qiagen, California, USA), amplification was conducted in triplicate in the Rotor-Gene Q 5plex HRM Instrument (Qiagen), and analysis was completed using Rotor-Gene Q Software Version 2.2.3. The 24μL final volume reactions included: 8μL of genomic DNA (0.2ng/μL), 2× Rotor-Gene SYBR Green PCR kit (Qiagen, Hilden, Germany), RNase-free water (Qiagen), and the primers Telomere Fw (CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT – 300nM) and Rv (GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT – 300nM) or single gene (36B4) Fw (CAGCAAGTGGGAAGGTGTAATCC – 300nM) and Rv (CCCATTCTATCATCAACGGGTACAA – 500nM). Telomere reactions were performed as follows: denaturation at 95°C for 5min followed by 25 cycles of 7s at 98°C and 10s at 60°C, whereas single gene reactions were denatured at 95°C for 5min followed by 35 cycles of 7s at 98°C and 10s at 58°C. The telomere length for each sample was determined using the telomere to single copy gene ratio (T/S ratio) with the calculation of ΔCt [Ct(telomere)/Ct(single gene)]. The T/S ratio for each sample (x) was normalized to the mean T/S ratio of the reference sample [2−(ΔCtx−ΔCtr)=2−ΔΔCt], which was used for the standard curve, both as a reference sample and as a validation sample. The acceptable coefficient of variations (CV) of triplicate measurements were 2% and 1% or less, for telomere and single gene reactions, respectively. The considered inter-assay CV was up to 5%.
Leukocyte telomere length by Southern blot analysisTo validate the qPCR terminal restriction fragment (TRF), analysis was performed by Southern blot of 17 samples according to the manufacturer's instructions with minor changes (TeloTAGGG Telomere Length Assay – Roche Applied Science, Mannheim, Germany). Briefly, 800ng of genomic DNA was digested by an optimized mixture of HinfI and RsaI FastDigest restriction enzymes (Thermo Scientific, Waltham, MA, USA) at 37°C for 2h. Following DNA digestion, DNA fragments were separated by electrophoresis in 0.8% agarose gel during four hours at 80V. Gel was denatured and neutralized, samples were transferred to a nylon membrane by Southern blotting and probed, and the terminal restriction fragments were detected by chemiluminescence. Mean TRF length was determined according to the formula TRF=Σ(ODi)/Σ(ODi/Li), where ODi is the chemiluminescent signal and Li is the length of the fragment at a given position.
Inflammation markersTumor necrosis factor-alpha (TNF-α) and IL-8 were assessed as pro-inflammatory markers. TNF-α and IL-8 levels were measured, in duplicate, in serum samples using ultra-sensitive enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (TNF-α US and IL-8 US, Invitrogen, Camarillo, CA, USA).
Statistical analysesThe relationship between telomere length and age was estimated using linear regression. Estimated regression coefficients were used to calculate the observed minus expected (O-E), or age-adjusted telomere length for each subject. Age-adjusted T/S ratios were used for all the study analyses except for the correlation with age. Spearman's rank correlation coefficient was used to analyze bivariate associations between telomere lengths, hemolysis and inflammation markers. T/S ratios were compared according to the diagnosis, use of hydroxyurea and gender using Wilcoxon rank sum test. Linear regression was used to analyze the correlation between telomere length measured by qPCR and TRF by Southern blot. All p-values ≤0.5 were considered significant. Statistical analyses were performed using the R statistics program version 3.1.3.
ResultsPatients’ characteristicsNinety-one adult SCD patients were included in the study: 51 Hb SS, 38 Hb SC, and 2 Hb Sβ+. Forty were on hydroxyurea with a median dose of 1g/day (range: 500–1750mg/day). The dose was titrated according to clinical improvement or to the maximum tolerated dose. The clinical indications for hydroxyurea were frequent painful crises (n=14; 35%), acute chest syndrome (n=5; 13%), stroke (n=6; 16%), and severe hemolytic anemia (n=15; 37%). Patients’ clinical characteristics are described in Table 1. The control group consisted of 188 healthy individuals (Hb AA), with a mean age of 38 years (range: 18–88 years), including 97 women and 91 men.
Clinical characteristics of sickle cell disease patients.
| Hb SS | Hb SC/Hb Sβ+ | |
|---|---|---|
| Number of patients – n | 51 | 40 |
| Mean age – years (range) | 37 (18–58) | 41 (19–66) |
| Gender: male/female – n (%) | 18/33 (35/65) | 14/26 (35/65) |
| α-Thalassemia trait* – n (%) | 11 (23) | 11 (32) |
| Use of hydroxyurea – n (%) | 37 (73) | 3 (8) |
| Mean age-adjusted T/S – ratio (range) | −0.34 (−0.61 to 0.21) | −0.21 (−0.51 to 0.68) |
T/S: telomere to single copy gene ratio.
Telomeres were significantly shorter in SCD patients compared to age-matched healthy controls [T/S ratio: −0.28 in SCD vs. −0.01 in controls; standard deviation (SD)=0.20 vs. 0.23; p-value<0.0001 – Figure 1A]. When genotypes were compared, the telomeres were significantly shorter in Hb SS patients than in the Hb SC and Hb Sβ genotypes (T/S ratio: −0.34 vs. −0.21, respectively; SD=0.17 vs. 0.21; p-value<0.0001 – Figure 1B). Telomeres were also shorter in patients on hydroxyurea (T/S ratio: −0.34 vs. −0.25; SD=0.20 vs. 0.20; p-value=0.02) (Figure 1C). Although telomeres shortened with age in healthy controls (r=−0.5; p-value<0.0001), age did not affect telomere length in SCD patients (r=−0.02; p-value=0.8). Telomere length was not influenced by gender in either SCD patients (p-value=0.2) or controls (p-value=0.9).
Age-adjusted T/S ratio in controls and sickle cell disease (SCD) patients. (A) SCD patients presented shortened telomere length compared to normal controls. (B) Hb SS patients presented shortened telomere length compared to Hb SC and Hb Sβ patients. (C) Hb SS and Hb SC patients treated with hydroxyurea (Hb SS/Hb SC HU) presented shortened telomere lengths when compared to patients not treated with hydroxyurea (Hb SS/Hb SC/Hb Sβ+). T/S ratios were compared according to the diagnosis and use of hydroxyurea using Wilcoxon rank sum test. Mean telomere length was measured in peripheral blood leukocytes by quantitative polymerase chain reaction (qPCR).
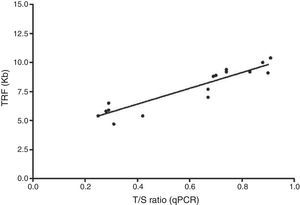
In order to validate these findings, telomere lengths were also measured by Southern blot for 17 patients showing that T/S ratios and TRFs were highly correlated (R2=0.86; p-value<0.0001 – Figure 2) supporting the accuracy of the qPCR results.
Correlation between telomere length measured by quantitative polymerase chain reaction (qPCR) and terminal restriction fragment (TRF) by Southern Blot. Analysis of 17 samples was performed to validate qPCR TRF. Telomere lengths measured by qPCR presented an adequate correlation with measurements of TRF by Southern blot, analyzed by linear regression (R2=0.86; p-value<0.0001).
The association between telomere length and marker levels for hemolysis (hemoglobin, hematocrit, lactate dehydrogenase, indirect bilirubin, reticulocyte counts) and inflammation (IL-8, TNF-α, total leukocyte, neutrophil, lymphocyte and monocyte counts) were analyzed (Table 2). There was a weak positive correlation between telomere length and hemoglobin concentration (r=0.3; p-value=0.004), but no correlation with other hemolysis markers. Among inflammatory markers, there was a weak negative correlation of telomere length with lymphocyte counts (r=−0.3; p-value=0.005) and with IL-8 serum levels (r=−0.4; p-value=0.02; Table 2). Of note, overall, SCD patients had higher lymphocyte counts (SCD: 2.9×109/L vs. controls: 1.9×109/L; SD=1.4 vs. 0.51; p-value<0.0001) and IL-8 levels compared to controls (SCD: 3.3pg/mL vs. controls: 2.3pg/mL; SD=1.9 vs. 0.8; p-value=0.009). Telomere length was not associated with the plasma levels of other inflammatory markers.
Correlations of telomere lengths with hemolysis and inflammation markers in sickle cell disease patients.
| Mean; median (range) | Correlation with telomere length (T/S ratio) r (p-value)a | |
|---|---|---|
| Hemoglobin (g/dL) | 9.8; 9.6 (6.0–16.0) | 0.3 (0.004) |
| Hematocrit (%) | 29.2; 29.1 (16.7–48.4) | 0.2 (0.2) |
| Lactate dehydrogenase (U/L) | 716; 615 (257–1680) | −0.2 (0.08) |
| Indirect bilirubin (mg/dL) | 2.2; 1.3 (0.6–9.0) | −0.2 (0.3) |
| Absolute reticulocyte count (×109/L) | 274; 261 (80–667) | −0.1 (0.3) |
| Absolute leukocyte count (×109/L) | 8.7; 8.6 (3.6–16.5) | −0.1 (0.3) |
| Absolute neutrophil count (×109/L) | 4.5; 4.6 (1.5–9.5) | 0.04 (0.7) |
| Absolute monocyte count (×109/L) | 0.5; 0.4 (0.1–1.4) | −0.03 (0.7) |
| Absolute lymphocyte count (×109/L) | 3.1; 2.9 (0.7–7.3) | −0.3 (0.005) |
| Absolute platelet count (×109/L) | 406; 407 (81–1164) | −0.2 (0.1) |
| TNF-α (pg/mL) | 2.6; 2.6 (0–6.9) | 0.07 (0.7) |
| IL-8 (pg/mL) | 3.5; 3.3 (0.8–11.6) | −0.4 (0.02) |
IL-8: interleukin 8; TNF-α: tumor necrosis factor-alpha.
In the present study, we found that telomeres of peripheral blood leukocytes of patients with SCD are short independent of age and telomere attrition was more pronounced in patients with Hb SS compared to Hb SC or Hb Sβ+ genotypes. Telomere length correlated with hemoglobin concentration and inversely correlated with IL-8 level and absolute lymphocyte count. Taken together, these findings suggest that telomere length is associated with disease severity and chronic inflammation in SCD.
Our results are in sharp contrast with one single previous report by Drasar et al., who found SCD patients had longer telomeres in comparison to age-matched controls.14 However, their study presented some technical issues precluding appropriate interpretation of their findings. First, it is not clear in their work whether they used fresh or frozen samples for DNA extraction. Second, telomere length was highly heterogeneous among their patients, and a significant proportion of older patients had telomeres longer than expected for healthy cord bloods. Finally, their qPCR results were not validated with a second method. Taken together, these concerns might suggest that the DNA used for analysis could have been degraded. During qPCR, DNA degradation causes T/S ratios to be higher and consequently telomeres to be erroneously interpreted as longer.10 This effect is explained by the significantly lower abundance (seven to eight log change) of the housekeeping gene (single gene) in comparison to telomere sequences. This difference makes the housekeeping gene more susceptible to DNA degradation, artificially augmenting the T/S ratio. In contrast, degraded DNA produces shorter telomeres in Southern blot due to DNA fragmentation. Thus, DNA degradation results in divergent results between qPCR and Southern blotting. To avoid this problem, all samples in our study were collected and processed within 16h from blood drawing for the purpose of telomere length measurement over a three-month period under rigorous quality control. To validate the qPCR findings of this study, telomere length was also performed by Southern blotting for 17 patients, with high correlation between both methods (R2=0.86). Analyzing the distribution of our patients simultaneously with ones from the English study, the ages of our patients were distributed more between 20 and 60 years old whereas the patients of the English study were mainly concentrated between 20 and 40 years old. In fact, the median age of our sample is almost ten years higher than the English sample (median age of Campinas vs. England – Hb SS: 39 vs. 32 years; Hb SC/Hb Sβ+: 43 vs. 34 years). Thus, we believe that divergences in sample number, disease severity, co-morbidities, socioeconomic status, ethnicity and age distribution of the patients and possibly DNA collection might be responsible for the conflicting results and encourage further studies to clarify this issue.
The involvement of white blood cells in the severity of clinical manifestations of SCD patients is well known.15–18 Indeed, the number of blood cells increases in SCD for many reasons, including high levels of circulating granulocyte macrophage colony-stimulating factor and increased cell survival.19–22
This study also observed that telomeres were shorter in patients using hydroxyurea. Hydroxyurea has a cytostatic effect and is capable of reducing the number of high turnover cells such as platelets, neutrophils, and reticulocytes. Hydroxyurea may have modulated blood counts, producing more prominent lymphocyte counts. In addition, most patients with more severe disease were on hydroxyurea, which may explain the association.
Telomere length did not correlate with age in SCD patients. As inflammation is chronic and starts at a young age, it may provoke excessive telomere shortening early in life, inducing early “aging” of the hematopoietic tissue in SCD patients. This finding may contribute to early multiple organ failure in SCD and may explain the shorter life expectancy related to the disease. This hypothesis should be addressed in future studies.
Our study has some limitations. More significantly, we recruited a small number of patients with the Hb Sβ+ genotype. In addition, we did not prospectively evaluate the effects of telomere length on disease complication events, such as acute chest syndrome and stroke. Finally, hydroxyurea may have interfered with telomere length by modulating leukocyte subsets.
In conclusion, our data confirm that telomere lengths are greatly influenced by inflammation and reactive oxygen species in SCD, a well-known phenomena of this disorder.
AuthorshipContribution: M.P.C. participated in the selection of patients, clinical follow up of patients, performed experimental work, analyzed the results and wrote the manuscript; B.A.S. participated in the selection of controls, performed experimental work and wrote the manuscript; N.C. and F.F.C. participated in the analysis of the results and writing of the manuscript; R.T.C. conceived the study, designed experiments, analyzed results and wrote the manuscript; S.T.O.S. conceived the study, participated in the selection of patients, clinical follow up of patients, analyzed the results and wrote the manuscript. All authors approved the final version of the manuscript.
Conflict of interestThe authors declare no conflicts of interest.
The authors would like to thank Roberto Zulli for his help with the statistical analyses.