Splenic marginal zone lymphoma (SMZL) is a low-grade B-cell non-Hodgkin's lymphoma characterized by massive splenomegaly, moderate lymphocytosis with or without villous lymphocytes, rare involvement of peripheral lymph nodes and indolent clinical course. As a rare disease, with no randomized prospective trials, there is no standard of care for SMZL so far. Splenectomy has been done for many years as an attempt to control disease, but nowadays it has not been encouraged as first line because of new advances in therapy as rituximab, that are as effective with minimal toxicity. Facing these controversies, this review highlights advances in the literature regarding diagnosis, prognostic factors, treatment indications and therapeutic options.
Splenic marginal zone lymphoma (SMZL) is a rare indolent non-Hodgkin lymphoma (NHL) subtype that originates from B memory lymphocytes present in the marginal zone of secondary lymphoid follicles.1–3
Patients usually present massive splenomegaly and bone marrow involvement with minimal or absent lymphadenopathy except for the spleen hilum. There is no extranodal involvement, except for the bone marrow and liver.3,4 About 25% of the patients are asymptomatic and the presence of B symptoms or high lactate dehydrogenase levels (LDH) at diagnosis is not usual.5,6
Lymphocytosis is commonly present. Cytopenias are found in 25% of the cases mostly related to hypersplenism, and less frequently to auto-antibodies or bone marrow infiltration.3,4
Small amounts (less than 2g/dL) of monoclonal protein, usually immunoglobulin (Ig)M kappa, are detected in approximately one third of patients.5,7 Hyperviscosity syndromes are not usual,3 but 20% of patients present autoimmune hemolytic anemia and other autoimmune disorders, such as thrombocytopenia, cold agglutinin disease, circulating anticoagulants and even angioedema because of acquired C1-esterase inhibitor deficiency.5,7,8
The rarity of this disease and its indolent course are a challenge to determine standard care in the treatment and management of patients. There are no randomized trials, most of the literature are retrospective series of cases from single centers and few prospective studies have been completed or are ongoing.8
EpidemiologySMZL is the second most common subtype of marginal zone lymphoma, comprising about 20% of the cases. It represents about 0.9% of all NHL and was considered a specific pathological entity only in 1991.1,5,9
Median age at diagnosis of SMZL is 69 years. The overall age-adjusted incidence is 0.13/100,000 habitants per year. The percentage change in age-adjusted incidence is 4.81%, with most of the patients being White.8 Gender prevalence is controversial,6,7 but there is an increasing trend to male predominance.8,10,11
The association of SMZL with hepatitis C (HCV) is common in the south of Europe,3,12,13 and lymphoma development is usually triggered by the glycoprotein E2 of the virus that stimulates CD81 in B cells.5,6,13 Although there are controversial data in Brazil regarding the association of HCV and lymphoma, no studies have evaluated this association.14,15
The International Lymphoma Epidemiology Consortium Non-Hodgkin Lymphoma Subtypes Project, with a database of 17,471 NHL cases and 23,096 controls, identified an association between SMZL and B cell activating autoimmune conditions, asthma and use of hair dye.16
DiagnosisThe diagnosis of SMZL can be by the analysis of pathological cells present in bone marrow with blood and spleen analysis not being essential.
Bone marrow infiltration is a very common finding (83–100%), although circulating cells are detected much less frequently (29–75%).7 During the course of the disease, 75% of the patients will present lymphocytosis, with characteristic, but not pathognomonic, villous cells.4,17 Bone marrow aspirate is not sufficient for diagnosis; a trephine histology with immunohistochemical analysis is required.5
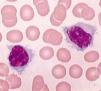
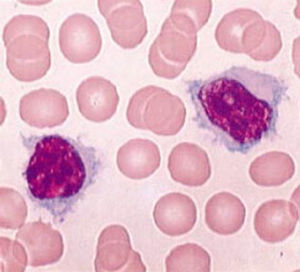
Pathological cells of SMZL are small- to medium-sized mature B cells with round or oval nuclei and condensed chromatin, basophilic cytoplasm, and most of the cases present with typical unequal membrane projections (villi), the so-called villous cells (Figure 1).4–7 Marrow infiltration can be nodular, interstitial or intrasinusoidal.5
There is no specific immunophenotypic pattern for SMZL. Pathological cells are usually positive for CD19, CD20, CD22, CD79a, CD79b, FMC7 and IgM and negative for CD5, CD10, CD43, BCL6, cyclin D1 or CD103. The expressions of CD23, IgD and cytoplasmatic Ig are variable,6,18 usually scoring 0–2 points in the Modified Matutes scoring system.19 CD5 are weakly positive in 10–25% of the cases, even with the co-expression of CD23 or CD43.20 CD11c and CD25 are sometimes positive, but CD103 and CD123 are almost always negative.4
Bone marrow immunohistochemistry analysis reveals positivity for CD45RA, CD45RB, CD19, CD20, CD79a, PAX5/BSAP, IgD, Bcl-2, DBA-44 (CD72), TRAP and CD38.5,21,22 IgM is usually bright, but IgD is variable.4,6 Cells are usually negative for CD3, CD5, CD10, CD23, CD43, cyclin D1, anexin-A1 and BCL6. KI67/Mib1 has a low proliferation index with a characteristic pattern.4,6
The spleen is frequently enlarged, with a median weight of 1750g (270–5500g) and many grayish nodules throughout the parenchyma.6 White pulp is expanded by neoplastic cells that surround and eventually substitute germinal centers. Nodules are composed of pathological cells, located in an inner zone of small- to medium-sized B cells with round nuclei, clumped chromatin and scanty cytoplasm. Externally there is an outer zone with medium-sized pathological cells, with more irregular nucleus outlines, dispersed chromatin and moderately clear cytoplasm. There are scattered cells in this zone resembling immunoblasts. As the disease progresses, the central germinal center becomes effaced. The red pulp is invariably enveloped to a varying degree by small aggregates of larger cells and sheets of small cells, which often occupy sinuses and cords. There can be epithelioid granulomas and plasmacytic differentiation, the former especially when there is a monoclonal serum component. Immunohistochemical findings are similar to bone marrow findings.5,6,9
Matutes et al. proposed minimum diagnostic criteria for SMZL:
- a)
When spleen pathology is available: spleen histology and immunophenotype with a modified Matutes score of <3 points.19
- b)
When the patient has clinical splenomegaly and splenectomy is not performed, it is sufficient to make the diagnosis with typical blood and bone marrow findings by morphology and immunophenotype with intrasinusoidal infiltration by CD20+ cells.
After the diagnosis is performed, it is important to evaluate the clinical stage of the patient, with computed tomography scans and routine exams to detect comorbidities that may affect the choice of treatment. These exams should include complete blood count with differential, serologic tests for hepatitis B and C and human immunodeficiency virus (HIV), renal and liver function tests, serum calcium, LDH, and b2-microglobulin. SMZL is regarded as a none F-18 fluorodeoxyglucose-avid disease; thus, the use of fluorodeoxyglucose positron emission tomography (FDG-PET) should be discouraged in the staging process.23
Differential diagnosisThe differential diagnosis requires the joint analysis of clinical, morphological, immunophenotypic and genetic data, as well as immunohistochemistry.5,6
Reactive follicular hyperplasia and other small B cell lymphomas should be excluded, as the pattern of splenic micronodular involvement of marginal zone differentiation and the villous lymphocytes in peripheral blood are not pathognomonic.4
A diagnostic test should not be performed on spleens weighing less than 300–400g or in the absence of a standard monotypic pattern.8
CD43 and CD200 positivity and a high (3–5) modified Matutes score helps to differentiate between SMZL and chronic lymphocytic leukemia.19 Intrasinusoidal infiltration is unusual in chronic lymphocytic leukemia, but often seen in SMZL, in hairy cell leukemia variant (LCP-v) and sometimes in mantle cell lymphoma (MCL).5 In rare cases of SMZL, CD5+, morphology, negativity for cyclin D1 and SOX11, and absence of t(11;14) excludes MCL.
Hairy cell leukemia (HCL) subtypes involving the spleen are distinguished by their characteristic morphology and phenotype. CD103 and CD123 negativity exclude HCL.4
Unlike SMZL, nodules have variable sizes and tumoral cells are seen in white pulp in the case of follicular lymphoma (FL). CD10 and BCL6 expression are useful for the diagnosis of FL. The morphological characteristics of the MIB 1 tumor cell staining pattern, residual mantle cell, IgD staining for tumor cells in addition to histological findings in bone marrow and hilar lymph nodes help establish diagnosis.5
Differential diagnosis between SZML, splenic diffuse red pulp lymphoma (SDRPL) and HCL-v can be tricky and sometimes impossible only by blood or bone marrow analyses. These are two newly recognized entities with clinicopathologic and immunophenotypic features partially overlapping those of SMZL. The diagnosis in these cases requires detailed clinical information, a comprehensive phenotype and spleen histology, which usually shows a typical pattern of diffuse infiltration with white pulp follicles preserved.8
An immunophenotypic profile with the absence of CD25, CD123, interleukin-3 anti-receptor, annexin A1, HC2 and TRAP and resistance to conventional HCL therapy is observed for HCL-v.6,24,25 Moreover, HCL-v is positive for the DBA-44, pan-B cells, CD11c, surface monotypic Ig (IgG most often) and CD103 FMC7.5,9
Although SDRPL have characteristics that overlap classic SMZL, the expression of IgD and the follicular micronodular pattern is absent in most cases.5 The distinction between those two entities may be merely academic, as the treatment is not different.26
Lymphoplasmacytic lymphoma (LPL) may develop in the spleen, with homogeneous infiltration of the white pulp without standard marginal zone and monocytoid B cells. Deletions of 7q, 3T gains and intrasinusoidal infiltration are characteristic of SMZL, while del(6q) is more characteristic of LPL.27 Another useful marker is the MYD88 L265P mutation, that is frequent in LPL (91–100%) and rare in SMZL (6%).28
Moreover, there are overlapping patterns of extranodal marginal zone lymphoma (EMZL) and SMZL with the clinical findings being crucial for differentiation. Splenic involvement is rare in nodal marginal zone lymphoma (NMZL) and Immunoglobulin Superfamily Receptor Translocation Associated 1 (IRTA1), negative in 76% of SMZL, is positive in NMZL.24 Two useful features to distinguish between SMZL and mucosa-associated lymphoid tissue (MALT) are the absence of the t(11;18)(q21;q21)29 and the frequent IgD expression in SMZL, which is rarely observed in MALT lymphoma.5,30
PrognosisAlthough most of the cases of SMZL have an indolent course with median overall survival of about ten years,18,22 about 30% of the patients develop aggressive disease, with median overall survival of only four years.10,18 There are no associated cytogenetic features31 and prognostic scores for indolent lymphomas such as the International Prognostic Index (IPI)18,32 and Follicular International Prognostic Index (FLIPI)33 are not applicable. The same can be said for the Ann Arbor staging system, which is not adequate because in most cases the bone marrow is involved.34
There are some clinical features associated with a worse outcome such as the development of lymphadenopathy, increase in β2-microglobulin, non-hematopoietic site involvement, leukocyte count >20×109/L, lymphocytosis >9×109/L, lymphopenia, anemia, thrombocytopenia, use of chemotherapy, monoclonal component, performance status ≥2, incomplete response, advanced age, diffuse pattern of bone marrow infiltration and histologic transformation.8,10,35–38
Many karyotype abnormalities can be found: trisomy 3q (85%) del or translocation of 7q32 (40%), trisomy 18, 17q isochromosome, 13q14 deletion, and structural abnormalities of chr 1.39 Some molecular aspects, such as NOTCH2 and KLF2 mutations, Ig gene mutation status, TP53 abnormalities and aberrant promoter methylation seem to be related to a worse outcome.31,37,40,41 Studies from a whole exome sequencing study identified the MYD88 L265P missense mutation in 15% of SMZL.42
The Italian Intergroup for Lymphomas (IIL), now Fondazione Italiana Linfomi (FIL) developed the first prognostic score after a multicenter trial with 309 patients. Specific event survival (SES) related to death by lymphoma was analyzed, as was overall survival. The 5-year cause-specific survival rate was 76%. The three most important parameters in multivariate analysis were hemoglobin <12g/dL, LDH higher than normal and low albumin (<3.5g/dL). Patients were grouped in three categories according to 0, 1 or 2–3 parameters, respectively, low risk (41% of the cases, 5-year SES of 88%), intermediate risk (34% of the cases, 5-year SES of 73%) and high risk (25% of the cases, 5-year SES of 50%).18 A recent study by Perrone et al. validated the score.26
In 2012, the Hemoglobin-Platelet-LDH-extra-hilar-Lymphadenopathy (HPLL) score was proposed by the SMZL Study Group after a retrospective analysis of 593 patients. Patients were stratified in three groups as shown in Table 1. The criteria of the IIL were applied to the same population but the stratification power for SES of the HPLL score were better,43,44 so this seems to be the most suitable score so far.
Indication for treatmentThere are no standard criteria to indicate treatment. The overall survival of asymptomatic patients can be as high as 88% at five years without treatment23.
Tarella et al.,23 proposed some criteria to indicate treatment (Table 2).
The SMZL Study Group also considered low hemoglobin levels, extranodal disease and a positivity for HCV as important to indicate treatment even though these factors have not been validated yet.44
A recent study by Perrone et al. suggested that patients should undergo an evaluation of the tumor burden similar to follicular patients, but this awaits further validation.26
Types of treatmentAs a rare disease with an indolent course, determining the standard treatment and management is a challenge as there have been no randomized trials and most reports are of single-center series of retrospective cases; few prospective trials have been completed or are ongoing.8 Therefore, nowadays there is no standard care for SMZL.
Therapeutic options for SMZL comprise splenectomy, chemotherapy and the use of the anti-CD20 monoclonal antibody rituximab alone or in chemotherapy combinations.35,45–52
SplenectomySplenectomy was the therapy of choice for decades and is still frequently used, although there is a tendency to prescribe rituximab monotherapy upfront, as most patients are old and with co-morbidities.10,11,52–54 Laparoscopy should be preferred whenever possible in patients with advanced age or comorbidities.8
Although marrow involvement is not treated, splenectomy allows quick remission of the symptoms of hypersplenism and cytopenias, such as a significant reduction of circulating lymphocytes in 90% of patients. Regarding clinical improvement, in a series report, seven patients (25%) had increases in bone marrow infiltration by pathological cells, there was a modification of the pattern in five of them.
The median overall survival in most series is about ten years and 70% of the patients can remain treatment free for five years.17,36,53 There is no survival benefit for the association of chemotherapy with splenectomy,17 although some studies report increases in overall response rates.47Tables 3 and 4 summarize the studies regarding different types of therapy for SMZL.
Splenic marginal zone lymphoma patients treated with splenectomy.
| Reference | Year | n | ORR (%) | Response | Death due to surgery | |
|---|---|---|---|---|---|---|
| Duration | OS | |||||
| Mulligan et al. | 1991 | 20 | 95 | Median DOR 4 years | NR | 1 |
| Troussard et al. | 1996 | 28 | 75 | NR | 71% at 5 years | 1 |
| Chacón et al. | 2002 | 60a | 93.3 | Median FFS 40 months | 65% at 5 years | NR |
| Thieblemont et al. | 2002 | 48b | 100 | PFS 48% at 5 years | NR | NR |
| Parry-Jones et al. | 2003 | 33 | NR | NR | LSS 95% at 10 years | NR |
| Iannitto et al. | 2004 | 21 | 91 | Median DOR 4 y | NR | NR |
| Tsimberidou et al. | 2006 | 10 | 60 | FFS 80% at 3 years | 89% at 3 years | 0 |
| Olszewski et al. | 2012 | 652 | NR | NR | 67.8% at 5 yearsc | NR |
| Kalpadakis et al. | 2013 | 27 | 85 | PFS 58% at 5 years | 77% at 5 years | 1 |
| Lenglet et al. | 2014 | 100 | 97 | PFS 61% at 5 y | 84% at 5 years | 0 |
| Xing et al. | 2015 | 52d | NR | FFS 39% at 10 years | 61% at 10 years | 0 |
| Pata et al. | 2015 | 41 | 90 | PFS 35% at 5 years | 75% at 5 years | 0 |
DOR: duration of response; FFS: failure-free survival; LSS: lymphoma-specific survival; NR: not reported; ORR: overall response rate; OS: overall survival; PFS: progression-free survival.
Splenic marginal zone lymphoma patients treated with rituximab-based regimens.
| Reference | Year | Study type | Regimen | Patient status | n | ORR (%) | Response | |
|---|---|---|---|---|---|---|---|---|
| Duration | OS | |||||||
| Rituximab monotherapy | ||||||||
| Bennett et al. | 2005 | Retrospective | R monotherapy | RR | 11 | 91% | PFS 60% at 5 years | 70% at 5 years |
| Tsimberidou et al. | 2006 | Retrospective | R monotherapy | First line | 25 | 88% | FFS 86% at 3 years | 95% at 3 years |
| Kalpadakis et al. | 2007 | Retrospective | R monotherapy | First line | 16 | 100% | PFS 92% at 2.4 years | 100% at 2.1 years |
| Else et al. | 2012 | Retrospective | R monotherapy | First line and RR | 10 | 100% | DFS 89% at 3 years | NR |
| Kalpadakis et al. | 2013 | Retrospective | R monotherapy | First line | 58 | 95% | PFS 73% at 5 years | 92% at 5 years |
| Rituximab+Chemotherapy | ||||||||
| Tsimberidou et al. | 2006 | Retrospective | R-chemo | First line | 6 | 83% | FFS 100% at 3 years | 100% at 3 years |
| Else et al. | 2012 | Retrospective | R-chemo | First line and RR | 33 | 100% | DFS 71% at 3 years | NR |
| Cervetti et al. | 2013 | Retrospective | R-2CDA | First line and RR | 47a | 87% | PFS 80% at 5 years | 86% at 5 years |
| Iannitto et al. | 2015 | Prospective | R-COMP | First line | 51 | 84% | PFS 54% at 6 years | 72% at 6 years |
2CDA: Cladribine; chemo: chemotherapy; DFS: disease-free survival; R: rituximab; COMP: non-pegylated lyposomal doxorubicin, cyclophosphamide, vincristine, prednisone; RR: relapsed/refractory; NR: not reported; ORR: overall response rate; OS: overall survival; PFS: progression-free survival; FFS: failure-free survival.
Pata et al. reported perioperative complications in one quarter of 41 patients submitted to splenectomy as first-line treatment: eight cases (19.5%) of pulmonary dysfunction, one case (2.4%) of deep vein thrombosis, one case (2.4%) of portal vein thrombosis and nine cases (22%) of major bleeding.55
Infections caused by encapsulated bacteria are the major risk associated with splenectomy and vaccination against capsulated bacteria is mandatory at least two weeks before elective splenectomy.8
Splenectomy should not be performed if the patient has nodal involvement outside the splenic hilum and, conversely, it should not be omitted in cases with suspected transformation.8
ChemotherapyAlkylating agents and purine analogs have been used as have many chemotherapy combinations such as cyclophosphamide, vincristine and prednisone (CVP); cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP), and fludarabine and cyclophosphamide (FC).35,45–51 About two-thirds of patients do not respond to first-line treatment with chorambucil.6
Rituximab monotherapyRituximab as monotherapy is effective in SMZL with results similar to splenectomy; it has the potential to provide better responses and has less toxicity compared to chemotherapy.8 Rituximab has little impact on the quality of life, has reduced risk of infections, seems to induce durable remissions and can be used again at relapse.46,49,50,56 Clinical and laboratorial responses are fast, with improvement in blood counts in about eight weeks.57
Some studies report inferior outcomes of rituximab monotherapy compared to splenectomy, but in non-randomized retrospective clinical trials there may be a bias of selecting younger and fitter patients for splenectomy (Table 3).8
Kalpadakis et al. reported a retrospective study of 58 patients treated with rituximab 375mg/m2 in an induction phase (weekly for six weeks) followed by a maintenance phase with rituximab every two months for one to two years. The complete response (CR) rate after the induction phase was 45%, unconfirmed CR was 26% and partial response was 24%. The 5-year overall survival and progression-free survival were 92% and 73%, respectively (p-value <0.001)46. There are other regimens using rituximab; weekly for four weeks with or without maintenance as reported by Bennet et al.58 The best regimen, whether to use maintenance or retreatment at relapse, is also areas that need to be clarified.
Rituximab with chemotherapyThe aforementioned chemotherapy options are used alone or with rituximab. Purine analogs are more toxic and should be reserved for refractory or relapsed cases. Fludarabine has high response rates, with CR in 70% of cases and progression-free survival of 4.7 years.57,58 A combination with Cladribine increased the CR from 21.4% to 62.5%, and four-year progression-free survival from 52.4% to 83.4%.51
There are no results from randomized trials specific for SMZL, but there are some ongoing studies, such as the BRISMA phase II trial with Bendamustin plus rituximab, NCT01332968 with obinutuzumab plus CHOP/CVP/BR, ibrutinib (NCT01980628 and NCT01974440), and PI3K inhibitors (NCT01282424, NCT01732926, NCT02369016, NCT02367040, and NCT01732913) (Table 4).
Treatment of patients with splenic marginal zone lymphoma and hepatitis CPatients with hepatitis C who do not require an immediate cytoreductive treatment should receive first-line antiviral treatment with pegylated alpha-interferon and ribavirin, because a CR of SMZL occurs in about 75% of the cases.39,59
Splenic irradiationSplenic irradiation has historical interest and there are isolated reports of its use before the era of rituximab therapy.21,60
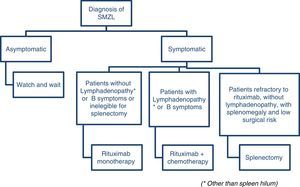
Treatment considerationsArcaini et al. proposes a consensus using the guidelines of both the European Society for Medical Oncology39 and the Società Italiana di Ematologia.23 According to the European Society, rituximab monotherapy is a reasonable first-line therapy and a less traumatic alternative to splenectomy and according to the Italian Society, rituximab is a good option for patients without disseminated disease (no lymphadenopathy other than spleen hilum, no constitutional symptoms or signs of high-grade transformation) who need treatment and are not eligible for splenectomy. The group of patients with constitutional symptoms or signs of high-grade transformation may be eligible for rituximab-chemotherapy combinations. There is no standard care so far, but combinations with CVP and chlorambucil are accepted as first line.23
Figure 2 illustrates a suggested algorithm for the treatment of SMZL patients based on these guidelines.
Response evaluationThe criteria used to evaluate response of patients with SMZL to treatment are shown in Table 5.
Response criteria for splenic marginal zone lymphoma.
| Complete response | • Resolution of organomegaly (spleen longitudinal diameter<13cm). • Hemoglobin >12g/dL, platelets >100×109/L, and neutrophils >1.5×109/L. • No evidence of circulating clonal B cells by flow cytometry (light chain restricted B cells). • No evidence of bone marrow infiltration detected by immunohistochemistry. • Optional: negative direct antiglobulin test (DAT) and normal positron emission tomography (PET) scan (if positive at diagnosis). |
| Partial response | • Regression of ≥50% in all the measurable disease manifestations. • No new sites of disease. • Improvement of cytopenias. • Decrease of infiltration and improvement of hematopoietic reserve at bone marrow biopsy. |
| No response | • <10% improvement of the disease manifestations. |
| Progression | • >50% of measurable signs of the disease from nadir. |
| Relapse | • Re-appearance of any measurable sign of the disease. |
Asymptomatic patients should be seen every six months with no more than a physical examination, blood counts, and biochemistry. The interval between visits should be shortened in cases of increasing splenomegaly or the occurrence of cytopenia. Computed tomography and bone marrow biopsy are not indicated unless signs of disease progression are identified.39
During the first three months of treatment, blood counts and laboratory work-ups should be performed every four to six weeks and every six months thereafter.39
Final considerationsSZML is an indolent lymphoma that presents many unsolved questions, such as standard prognostic criteria and standard treatment. As it comprises less than 2% of lymphomas, large randomized clinical trials are not likely and review articles that clarify some issues are important in the clinical practice.
Conflicts of interestThe authors declare no conflicts of interest.