Anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphomas (ALCLs) are recognized as a distinct clinicopathologic entity in the World Health Organization (WHO) classification of hematopoietic neoplasms.1 Based on the morphologic features, mainly characterized by anaplastic large cells, and on the expression of the Ki-1 antigen (CD 30), it was first described in 1985.2 From 1990 onwards studies have shown that many ALCLs are associated with the t(2;5)(p23;q35) responsible for the upregulation of the ALK protein. ALCLs account for 2–8% of non-Hodgkin lymphomas in adults and approximately 10–30% of lymphomas in children; the ALK-positive (ALK+) ALCLs largely occur within the first three decades of life.3 Morphologically, ALK+ ALCLs are composed of a variable amount of so called ‘hallmark cells’ illustrated by large elements with eccentric, horseshoe- or kidney-shaped nuclei. These cells can be admixed with smaller lymphocytes, Hodgkin and Reed-Sternberg-like neoplastic cells as well as with reactive inflammatory cells, resulting in a broad range of histologic patterns recognized by the WHO as common, lymphohistiocytic, small cell and Hodgkin-like types, though composite or other unusual types can be encountered.1 Most patients (70%) present with advanced stage disease and systemic symptoms (75%), particularly high fever. Extranodal involvement is common and mainly comprises skin, bone, soft tissue, lung and liver.3–5 We herein report a case of an adult woman presenting with rapidly progressive neoplastic small cell lymphocytosis: only rare cases with leukemic presentation have been described.6–14
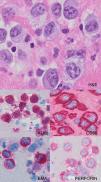
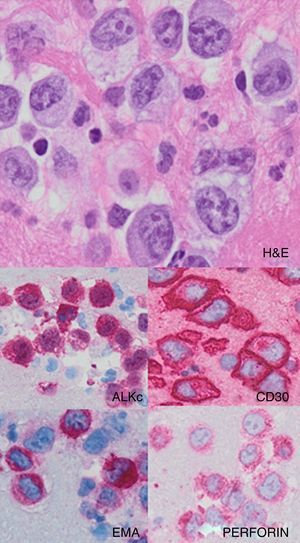
Case reportA 37-year-old woman presented with a three-week history of fever, asthenia, night sweats, and dyspnea. Imaging studies with computed tomography (CT) revealed slightly enlarged superficial and deep lymph nodes, the largest one of which was 35mm in diameter at an inguinal site. The craniocaudal length of the spleen was 14.5cm. The complete blood count revealed leukocytosis (39.0×109/L) with rapid doubling time (80.0×109/L after one week) with marked lymphocytosis (90%) that showed a T-cell phenotype at flow cytometry (positivity for CD3, CD4, CD5, CD7, and CD2 and negativity for immature markers). Molecular studies revealed T-cell receptor rearrangement. She underwent a bone marrow biopsy that showed a mild nodular and interstitial infiltration by small lymphocytes with slightly irregular nuclear contours positive for CD3, CD4, CD2, CD5, and CD7 molecules and negative for CD20, CD8 and TCL1. The results were interpreted as TCL1 negative T-cell prolymphocytic leukemia (TPLL). After one cycle of chemotherapy, the patient received the human anti-CD52 antibody that had to be discontinued due to a severe respiratory insufficiency likely secondary to cytomegalovirus infection which responded well to corticotherapy and specific therapy. A similar episode was observed nearly one month after therapy reintroduction, with fever, progressive worsening of clinical symptoms and pancytopenia. Peripheral blood flow cytometry detected 4% of residual neoplastic cells and a higher CD8+ lymphoid component that was interpreted as a virus-mediated reaction and supported by cytological evidence of large “lympho-mononuclear” circulating cells. A second bone marrow biopsy was unable to confirm residual disease while showing richness in myeloid precursors, plasma cells and some CD8+ cells that confirmed what was observed in the peripheral blood. Some weeks later, she developed ascites and, on cytologic examination, large cells with clear cytoplasm, irregular horseshoe-shaped nuclei and conspicuous nucleoli could be observed. Immunocytochemical tests were positive for CD30, CD4, EMA, PERFORIN and ALKc and negative for CD3, CD8 and PAX-5 (Figure 1). The results were interpreted as an ALK+ anaplastic large cell lymphoma and the patient started chemotherapy (methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin – MACOP-B scheme) with improvement of the clinical conditions. Neither lymph node enlargement at CT nor residual tumor in the bone marrow were detected. No molecular detection of the t(2;5) was carried out. Although this analysis was not performed, ALK staining, both nuclear and cytoplasmic, as seen in this case, usually correlates with the t(2;5) translocation involving ALK and NPM. Variant translocations will display membranous or cytoplasmic staining.15,16 Based on these findings we reviewed the first bone marrow biopsy performing additional immunohistochemistry for the ALKc protein which revealed positivity in some of the CD4+ small lymphocytes as well as in rare dispersed previously unrecognized atypical large cells which also turned out to be CD30+. This prompted a diagnosis of peripheral blood and bone marrow involvement by a small cell component of an ALK+ ALCL of the composite variant (showing common type features in the serosa) presenting in leukemic phase (Figure 2).
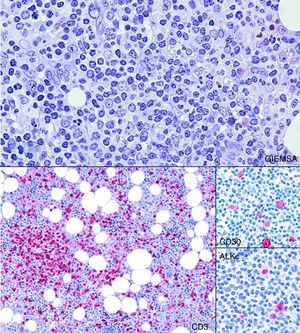
Histologic section from the bone marrow biopsy sample showing mild nodular and interstitial infiltration by small lymphocytes (Giemsa: 200×). CD3 highlights these lymphocytes (100×). CD30 and ALKc are detected by immunohistochemical staining in some of the atypical lymphocytes and become of essential importance to the differential diagnosis with T-cell prolymphocytic leukemia (400×).
ALK+ ALCL is a T-cell lymphoma characterized by cells that are generally large with abundant cytoplasm and pleomorphic, often horseshoe-shaped nuclei, wide CD30 expression and with a translocation involving the ALK gene which leads to overexpression of the ALK protein.1 Nodal and extranodal sites are commonly involved, with approximately 40% of patients showing two or more extranodal sites of the disease.3 The small cell variant of ALCL, first reported by Kinney et al. in 1993,17 accounts for 5–10% of cases and more often occurs in pediatric ages.1 This variant, which displays a predominance of small cells admixed with rare sparse medium/large elements3 has also been reported in association with the other histologic variants, such cases corresponding to the ‘composite type’ ALCL.10 Although CD30 expression is a defining characteristic of all ALCL variants, it characteristically shows a lower degree of intensity in the small cell component as compared to the strong staining on medium and large size cells; immunopositivity for ALK is present in all reported cases of small cell variant ALCL.18
Leukemic presentation in ALCL is highly unusual but most often reported in the small cell variant cases.9,18 So far a total of 26 cases of leukemic ALCL have been identified, mostly presenting with an elevated white blood cell count (average <100×109/L).6,8–10,12–14,17,19–28 Clinically, most patients presented with B-type symptoms, with fever being reported most (85.18%). The age of affected patients ranged from seven months to 63 years, but with 70% being younger than 30 years of age. There is a slight male predominance (55.5%).9,13,26–28 As observed by Nguyen et al. most cases (85%) of leukemic phase ALK+ ALCL showed the t(2;5)(p23;q35) translocation, although this observation cannot be regarded as statistically significant since t(2;5) occurs in approximately 80% of ALK+ ALCL regardless of the variant type.9
The diagnosis of a small cell variant ALCL in adults can be challenging6,18,29 particularly in the absence of a large cell component since it can also be mistaken for an infectious or inflammatory process. In fact, although the neoplastic cells do show irregular nuclear contours, the small size and the inconspicuous nucleoli are often misleading. In this case, the massive lymphocytosis and minor organomegaly prompted clinicians to search for diagnosis in the bone marrow biopsy where the infiltrate was quantitatively mild, organized in rare foci and in singly scattered small size cells without frank cytological atypia. Considering the clinical presentation, a TPLL and a large granular lymphocyte lymphoma/leukemia were considered as possible diagnoses: upon CD4 positivity a small cell variant TPLL1 was favored in spite of few unusual features such as negativity for TCL1 and the small degree of marrow infiltration. The presence of lymphadenopathies was not contrasting. The scattered large T cells could not be recognized in hematoxylin and eosin (H&E) stained sections, but were subsequently highlighted by their CD30 expression. Moreover, the presence of ‘activated’ lympho-mononuclear cells with CD8+ T-lymphocytes in the peripheral blood during the second viral infection could have justified the detection of few scattered CD30+ activated large cells in the bone marrow within an anti-viral CD8 mediated immune response. Our case was even more challenging since the cytological analysis of the circulating neoplastic cells did not show ‘flower-like’ nuclei or ‘hallmark’ features as sometimes reported in published cases.9 Only the later development of an ascites, where the large cell component was detected, allowed the correct classification of the process and the prompt improvement of the patient's clinical conditions upon the administration of a more appropriate therapy. Although most of the reported cases of leukemic ALK-positive ALCL seem to be associated with poor prognosis,13,14 some of them can present complete response to chemotherapy and/or bone marrow transplantation with variable disease-free periods. Onciu et al. report a case of a 6-year-old girl who was free of disease 17 months after diagnosis at the time they had written their manuscript.14 Our patient is at present doing well in complete remission.
ConclusionOur case confirms the high variability in clinical presentation of ALCLs which may have uncommon features, and should make hematologists and pathologists aware of the importance of taking a small cell variant ALCL into consideration also in adults, when faced with unusual T-cell lymphocytosis and/or TPLL-like presentations.
Conflicts of interestThe authors declare no conflicts of interest.