Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare acute leukemia subtype characterized by clonal expansion of dendritic-lineage cells.1 These cells are identified immunophenotypically by weak CD45 expression and co-expression of the CD4 and CD56 antigens in the absence of other lineage-specific markers.2,3 Previously known as blastic natural killer (NK)-cell lymphoma or CD4+/CD56+ hematodermic neoplasm, it is currently classified by the World Health Organization (WHO) as a distinct entity, under the acute myeloid leukemia (AML) and related precursor neoplasm group.4
Few studies have assessed the incidence of BPDCN in the general population. The limited existing data suggest an extremely low overall incidence, representing 0.44% of all hematological malignancies3,5 and 0.7% of cutaneous lymphomas.6 BPDCN mostly affects men, with a 3:1 male-to-female ratio, and usually occurs in patients aged 60–70 years7,8 although cases have been reported in children and young adults.9
BPDCN is particularly difficult to diagnose because of its clinical and biological heterogeneity that overlaps with other clinical malignancies, and frequent lack of chromosomal abnormalities.5,10 On laboratory testing, the cell morphology can be misleading, with a pseudolymphocytic appearance, abundant cytoplasm with a low nuclear-cytoplasmic ratio, strong basophilia, and no granulation. Microvacuoles are often visible, possibly representing glycogen compounds inclined to form a “pearl necklace” on the nuclear membrane, as well as cytoplasmic extensions similar to pseudopodia.11,12 The cell lineage must be evaluated by flow cytometry immunophenotyping, which identifies dendritic cells in their three stages of differentiation.10
A recent study showed that, according to their CD34 and CD117 expressions, dendritic cells can be categorized into three maturational stages: (1) in BPDCN, CD34 is expressed in some of the immature blasts; (2) intermediate cells are partially CD117-positive when the expression of CD34 is absent in blast cells; and (3) mature cells do not express CD34 or CD117. These stages of maturation explain the variation in the clinical presentation of BPDCN, as well as its laboratory characteristics.10
The most differentiated stage is characterized by cells with weak expression of CD45, no expression of CD34, co-expression of CD4/CD56, presence of HLADR/CD123, and absence of specific markers of myeloid, B and T lymphoid, and NK cells.13,14 Co-expression of CD2, cytoplasmic CD3, CD5, CD7, CD33, nTdT, CD79a, and/or CD117 can be common.13,15,16
CD123 is the α chain of the interleukin-3 receptor, and is a sensitive and specific marker of plasmacytoid dendritic cells (pDC). However, although CD123 is currently the most important marker in the diagnosis of BPDCN, it is not unique to the plasmacytoid dendritic cell lineage. CD4 and CD56 are expressed in other hematological malignancies, and are not enough to establish diagnosis.17,18
Clinically, this disease manifests with isolated cutaneous involvement in the form of single or multiple lesions, and, despite initial indolent behavior, it is characterized by aggressive, rapid systemic dissemination.19,20 Many patients have cytopenia, particularly thrombocytopenia, as a result of extremely variable rates of dendritic-cell infiltration of the bone marrow. Although there is an initial response to systemic chemotherapy, the disease relapses as a matter of course, and survival ranges from 12 to 14 months.19
Currently, there is no consensus as to the ideal treatment for BPDCN.1,21 Retrospective studies have evaluated different treatment strategies, including multi-agent chemotherapy according to established protocols for acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML), while a few cases have been submitted to allogeneic hematopoietic stem cell transplantation (HSCT).1,21 The neoplastic cells are initially sensitive to chemotherapy agents which are typically active against lymphoblasts, such as steroids, vincristine, and asparaginase. Therefore, it is recommended that ALL protocols be followed, with subsequent HSCT when appropriate.1,20 It is important to highlight that the effectiveness of this procedure still needs to be investigated by clinical studies, and the role of transplantation has yet to be defined.1 The Martín-Martín et al. study demonstrated that this strategy (ALL therapy plus HSCT) was associated with the best prognosis.10
Within this context, the aim of this paper is to report a case of this rare, difficult-to-diagnose neoplasm in which flow cytometry immunophenotyping contributed to the correct identification of leukemic cell lineage.
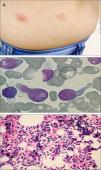
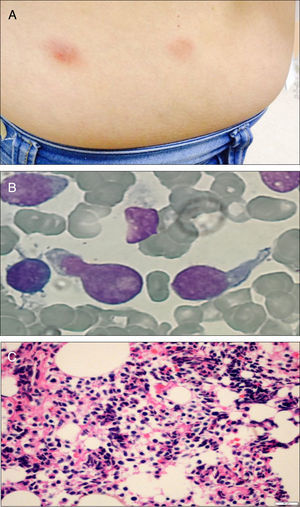
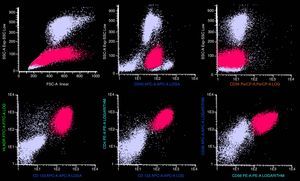
Case reportA 51-year-old previously healthy female presented with a 3-month history of progressively disseminating cutaneous lesions, with no defined diagnosis and no clinical response to topical treatments. Immunohistochemistry skin biopsy was performed and revealed blast-appearing cells, positive for CD45 and CD123, and negative for TCL1 related to the CD3 and CD20 antigens, indicating BPDCN. Bone marrow aspiration and biopsy did not show bone-marrow involvement at the time of diagnosis. Treatment was performed with cytarabine and daunorubicin (7+3 protocol – induction, re-induction, and three consolidations). Three months after completing high-dose cytarabine maintenance, a cutaneous relapse was detected (Figure 1A) with central nervous system (CNS) involvement. At this time, bone marrow biopsy identified 80% immature cells with lymphoblast appearance (Figure 1B) and an anatomopathological study revealed diffuse infiltration by blast cells of lymphoid origin (Figure 1C). Bone marrow immunophenotyping by flow cytometry (Figure 2) using the FACSCanto II system (Becton Dickinson, San Jose, CA, USA) showed 62% of cells with expression of the antigens CD4, HLADR, CD38, weak and heterogeneous CD56 expression, strong and homogeneous CD123 and weak CD45 expression. There was no expression of CD34 or markers of lymphoid B (cCD79a, CD19), lymphoid T (CD7, CD3, CD5, CD2), NK (CD11b, CD16), or myeloid cells (cMPO, CD13, CD15, CD64, CD65) suggesting BPDCL. The patient began a HyperCVAD regimen (cyclophosphamide, vincristine, doxorubicin and dexamethasone) and underwent allogeneic HSCT. The post-transplant course was complicated by septic shock and hemorrhagic alveolitis, and the patient died 18 days after HSCT.
BPDCN is rare, aggressive, and difficult to diagnose. Knowledge about this condition has been improving in recent years because of the increasing number of reported cases and a better comprehension of its biological characteristics.22 Given the clinical and biological heterogeneity of this neoplasm, correct identification and classification require the experience of the clinician and histopathologist, as well as appropriate diagnostic tests.
Diagnosis of BPDCN by flow cytometry may be challenging in some cases because of the different maturational stages or because cells do not exhibit the distinctive profile due to either the absence of the main markers or the presence of additional markers indicative of other lineages.10,13 The advantage of flow cytometry in the diagnosis of BPDCN is that it is a quantitative and qualitative method, in addition to being more sensitive and specific, as it not only demonstrates antigen co-expression but also shows the density of each antigen quantitatively. This technique also has better ability to detect numerous antigens simultaneously, including some not routinely tested by immunohistochemistry.12,23
Expression of CD123 is typical of BPDCN, but this marker can be found in basophils and in many cases of acute leukemia of other lineages.24 Intensity of expression of this antigen can vary during dendritic cell maturation, although strong, homogeneous expression is an important indicator of differentiated plasmacytoid dendritic origin; therefore, it must be evaluated together with other markers.13
It is important to distinguish BPDCN from other malignancies that express CD4 and CD56, such as aggressive NK-cell leukemia/lymphoma, nasal NK-cell lymphoma, and AML, which can feature aberrant expressions of these markers and cutaneous involvement.25
We reported the case of BPDCN in a female patient with an indolent clinical presentation, cutaneous and CNS infiltration, no initial bone marrow involvement, and no response to AML treatment. During relapse, a high percentage of plasmacytoid dendritic-lineage cells was identified in the bone marrow by flow cytometry. The patient died 18 days after HSCT.
We emphasize the importance of performing flow cytometry with a panel of monoclonal antibodies suitable to identify BPDCN, especially in patients presenting with lesions and neoplastic cells showing morphological characteristics indicative of the lymphoid lineage. This technique can establish the correct diagnosis and define the most appropriate treatment.25
FundingHCPA Research and Event Promotion Fund (FIPE-HCPA).
Conflicts of interestThe authors declare no conflicts of interest.