To recognize the profile of platelet donors and the profile of the plateletpheresis session as well as to investigate the main adverse events of platelet donation using plateletpheresis and associated risk factors.
MethodsThis retrospective, cross-sectional and analytical study was performed with a quantitative approach by analyzing 316 donation files from February 2010 to December 2011. The IBM SPSS Statistics program was used for data processing and analysis. The chi-square test was used to verify whether there was an association between factors related to the procedure and the donor, and the adverse events that occurred.
ResultsThe mean age of platelet donors was 40 years old (standard deviation=8.9), with the prevalent age group being between 40 and 49 years old; the prevalent blood type was O positive (53.8%), the mean duration of the procedure was 73min and the mean amount of anticoagulant used was 360mL. The association between procedure duration and the volume of anticoagulant was inverse and statistically significant; the longer the procedure and the greater the volume of anticoagulant used, the less adverse reactions occurred.
ConclusionThe low incidence of adverse events indicates that the procedure is well tolerated by donors. Obtaining data regarding the incidence of adverse events is a way of promoting a dynamic review of medical and nursing teams to improve the safety and comfort of the donor.
It is possible to collect the therapeutic unit required for a transfusion in an adult patient from a single donor using plateletpheresis. This reduces the risk of immediate transfusion reactions and disease transmission by blood transfusion.1
The increase in medical and surgical indications for platelet transfusion, along with the new technologies available, promoted and increased the use of plateletpheresis.2
Plateletpheresis should be performed in a specific area and under the guidance and supervision of a physician.3
The plateletpheresis procedure is considered relatively safe. However, several complications may occur. Anticoagulant (ACD) intoxication, which is due to hypocalcemia, entails perioral paresthesia of the extremities, tremors, dizziness, chills, and uncoordinated involuntary movements. Vasovagal reaction, which is characterized by pallor, sweating, nausea, hypotension, fainting and loss of consciousness is also a possible complication. Hypovolemia and bruising, which may be related to venipuncture as well as the use of a tourniquet on the arm for a long time and the continued movement of the hands, is also another possible complication.4
This study was proposed due to the lack of national studies on the issue and the need to understand the risk factors related to adverse events in platelet donation. This study was also proposed to provide support for the adoption of measures to prevent the occurrence of adverse events, minimizing the impact for the service and for the donor.
The aim of this study was to identify the profile of platelet donors and the profile of plateletpheresis donations as well as the major adverse events resulting from plateletpheresis donation and associated risk factors.
MethodsThis is a retrospective, cross-sectional and analytical study with a quantitative approach. It was developed in the Uberaba Regional Blood Center (Minas Gerais), a unit belonging to Fundação Hemominas, which collects a total of 14,000 bags of blood and 180 platelet collections by plateletpheresis annually. The Blood Center meets the demand of eight cities in the region through transfusion agencies and ten hospitals located in the city with blood treatment centers.
Once the research proposal was approved by the Research Ethics Committee at Fundação Hemominas (protocol no. 321/2011), an analysis was performed of 316 plateletpheresis and donation files, which had been stored in the unit between February 2010 and December 2011. An instrument, used to guide data collection, addressed clinical and epidemiological aspects, characteristics of plateletpheresis donations and donors, and the complications that occurred during the procedure, which were reported by the donor and noted by nursing staff, as per the institutional protocol for recording adverse events during donation.
The collection of platelets by plateletpheresis in the Blood Center is performed using a mobile platelet collection system (Haemonetics MCS®) and meets the following criteria: single units must contain at least 3.0×1011 platelets in at least 90% of the units evaluated and double units must contain at least 6.0×1011 platelets; the platelets must be valid for up to five days.
Donors are invited by the recruitment department to donate platelets using the plateletpheresis method. The donor, in order to donate platelets using this system, must have a platelet count of 150×109/L for single units and 250×109/L for double units.6
The classification of adverse events was based on the clinical manifestations presented by the donor. The following criteria were used:
- (1)
Mild clinical complications – syncope, malaise, dizziness, sweating, paresthesia, headache and paleness;
- (2)
Moderate medical complications – symptoms with a mild reaction, nausea, vomiting, hypotension and arrhythmia;
- (3)
Severe medical complications – the donor has symptoms of a mild to moderate reaction, hyperventilation, tetany, apnea, loss of consciousness and convulsive crisis,5 and hematoma.
The data collected were entered into the Excel® spreadsheet program (Windows® XP), by double entry for subsequent validation. The SPSS computer program (version 17.0) was used for data processing and analysis. Clinical and epidemiological variables were analyzed using descriptive statistics (absolute, relative and mean frequency).
The chi-square test was used to check whether there was an association between the independent variables (donor weight, gender, age, duration of the procedure and volume of ACD used) and adverse events occurring during the donation process (dependent variable). The logistic regression test was used for multivariate analysis. All results with an alpha error of 5% (p-value<0.05) were considered significant.
The analysis of the multiple logistic regression test consists of a multivariable dependency test, i.e., in order to employ it, the researcher must define a qualitative outcome or variable answer (adverse events) and a set of quantitative explanatory or categorical variables (risk factors) which relate to those variables. The result is the Odds Ratio adjusted for the risk factors considered in the study.7
ResultsIn terms of donor profile, 310 (98.1%) donors resided in Uberaba, 244 (77.2%) were male, and 162 (51.3%) were married. The mean age was 40 years [standard deviation (SD)=8.9], with the most prevalent age group being between 40 and 49 years old (40.8%).
The mean donor weight was 78.7kg; the mean donor height was 169cm; and the mean donor blood volume was 5084mL.
The prevalent blood type was O positive, which accounted for 53.8% of the donations. The mean values of hemoglobin and hematocrit before donation were 14.8g/dL and 44%, respectively.
The mean duration of a plateletpheresis session was 73min. The mean amount of platelets estimated for collection was 3.47×1011, whereas the mean number of platelets actually collected was 3.6×1011.
The mean volume of blood processed by the equipment was 2829.8mL, and the mean volume of the product obtained was 299.55mL. The mean amount of ACD used during the procedures was 360mL.
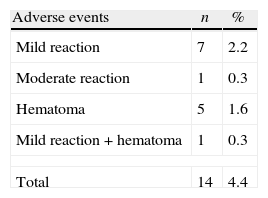
As shown in Table 1, a total of 14 (4.4%) donors had some type of adverse event: seven (2.2%) had mild reactions, one (0.3%) had a moderate reaction, five (1.6%) had hematomas, and one (0.3%) had a mild reaction associated with hematoma.
Among the donors who presented complications, the mean number of donations made by these individuals was 8.86 (SD=8.9). Among the donors who suffered adverse events, two (8.7%) were first time platelet donors. Among donors who had donated up to 10 times, eight (5.8%) had adverse events, and of the donors who had undergone plateletpheresis donation over 11 times, four (2.8%) had adverse events.
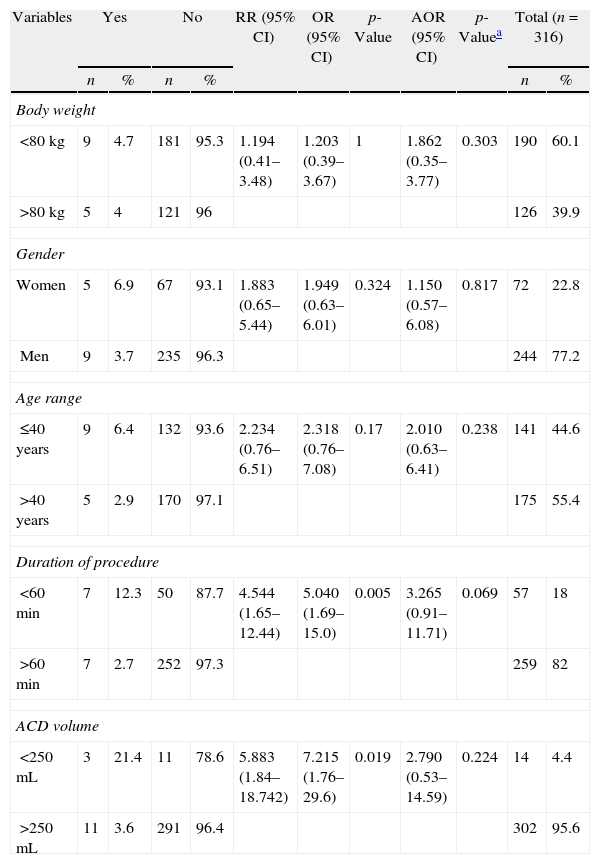
The bivariate and multivariate analyses, presented in Table 2, show a statistically significant association between the duration of the procedure (less than 60min) and the volume of ACD infused (less than 250mL) with the occurrence of adverse events (p<0.05). Nevertheless, adjusting for the other variables, the volume of ACD was not statistically significant, and the association with the time variable was marginally significant (p=0.069).
Association between the adverse events identified and the variables related to the donor and plateletpheresis procedure. Uberaba, 2010–2011.
| Variables | Yes | No | RR (95% CI) | OR (95% CI) | p-Value | AOR (95% CI) | p-Valuea | Total (n=316) | |||
| n | % | n | % | n | % | ||||||
| Body weight | |||||||||||
| <80kg | 9 | 4.7 | 181 | 95.3 | 1.194 (0.41–3.48) | 1.203 (0.39–3.67) | 1 | 1.862 (0.35–3.77) | 0.303 | 190 | 60.1 |
| >80kg | 5 | 4 | 121 | 96 | 126 | 39.9 | |||||
| Gender | |||||||||||
| Women | 5 | 6.9 | 67 | 93.1 | 1.883 (0.65–5.44) | 1.949 (0.63–6.01) | 0.324 | 1.150 (0.57–6.08) | 0.817 | 72 | 22.8 |
| Men | 9 | 3.7 | 235 | 96.3 | 244 | 77.2 | |||||
| Age range | |||||||||||
| ≤40 years | 9 | 6.4 | 132 | 93.6 | 2.234 (0.76–6.51) | 2.318 (0.76–7.08) | 0.17 | 2.010 (0.63–6.41) | 0.238 | 141 | 44.6 |
| >40 years | 5 | 2.9 | 170 | 97.1 | 175 | 55.4 | |||||
| Duration of procedure | |||||||||||
| <60min | 7 | 12.3 | 50 | 87.7 | 4.544 (1.65–12.44) | 5.040 (1.69–15.0) | 0.005 | 3.265 (0.91–11.71) | 0.069 | 57 | 18 |
| >60min | 7 | 2.7 | 252 | 97.3 | 259 | 82 | |||||
| ACD volume | |||||||||||
| <250mL | 3 | 21.4 | 11 | 78.6 | 5.883 (1.84–18.742) | 7.215 (1.76–29.6) | 0.019 | 2.790 (0.53–14.59) | 0.224 | 14 | 4.4 |
| >250mL | 11 | 3.6 | 291 | 96.4 | 302 | 95.6 | |||||
ACD: anticoagulant; RR: relative risk; 95% CI: 95% confidence interval; OR: odds ratio; AOR: odds ratio, confidence interval of logistical regression.
The potential donor has to meet several requirements to be accepted as a suitable candidate for blood component donation.6 Criteria such as hematocrit or hemoglobin levels, age, weight and minimum platelet count are important for the safety of the donor.5 Weight or body mass is indicated as criteria to maximize the donation of plateletpheresis, because larger donors have higher platelet yields due to the higher volume of blood.8
Several studies show a common profile for donation, which is a larger number of male donors.8–12 Some studies also show that men have lower rates of adverse events compared to women in plateletpheresis donation. The findings of this study are consistent with the literature.
In an investigation that evaluated the performance of two plateletpheresis devices, the data on the performance of Haemonetics mobile platelet collection system MCS 3p were a mean duration of 74.5±3.12min, volume processed 3.2–3.4L and volume of ACD used 330mL.13 This data corroborates the results of the current study, considering that similar equipment was used in the donation center. Making sure that the results of the equipment involved in the donation process are similar is essential to provide maximum safety to the donor and avoid complications due to faulty instruments.
Results showed that 14 donors (4.4%) had some type of adverse event. This low incidence is consistent with the literature, which indicates that the plateletpheresis procedure is well-tolerated by donors.9 Nevertheless, of apheresis donations, there are reports that adverse events are more frequent in plateletpheresis compared to other types such as plasmapheresis and leukapheresis.14
One complication, local bruising, was also described in another study.14 The significant presence of bruising as an adverse event is considered a non-random event15 because factors such as the experience of the professional performing the puncture, the number of prior apheresis donations, the anatomy at the venipuncture, the equipment used and the diastolic blood pressure are significantly correlated to the development of bruising. Unlike citrate reactions, which are more likely to occur in individuals who donated many times, the probability of bruising reduces with the number of donations.14,16
Other predominant clinical manifestations presented in the literature are a tingling feeling, numbness, muscle cramps, tetany or convulsions from reactions to citrate. Vasovagal reactions, which manifest as paleness, weakness, nausea, dizziness, vomiting, hypotension, tachycardia, bradycardia, or fainting, are also possible adverse effects.9 Most of these symptoms generally occur as moderate to severe adverse events. Nevertheless, in this study, although some of these symptoms were observed, mild adverse events were the most common.
Despite the low incidence of adverse events, studies indicate that women are 2.43–2.8 times more affected by moderate to severe events than men in apheresis donations,9,10 figures that are similar to the results in this study; even though the difference was not significant, 6.9% of the women had adverse events compared to 3.7% in men.
In contrast, another study found that the female gender is one of the factors independently associated with the risk of citrate toxicity and hypotensive events (non-vasovagal) in apheresis donations. Other factors associated with the risk of citrate reactions included the height of the donor and the model of the apheresis machine used. Other factors associated with the risk of hypotension included the height of the donor, plasma collection and the model of the apheresis machine. The results in this study also point out that only women were associated with complications related to the venipuncture.17
The present study indicated a marginally significant association between the shorter duration of the procedure and adverse events, with no association related to the volume of ACD. These findings differ from a study that shows that donors who undergo the procedure repeatedly or for prolonged periods are susceptible to an accumulation of citrate as levels exceed the amount that can be metabolized by the body.16 Another study revealed that adverse events occurred in apheresis procedures which took more time (mean 77.1min), and had a lower infusion of ACD (mean 301.5mL) compared to those without adverse events.10
It was not possible to establish causal relations with the findings of this study. Because of the retrospective and cross-sectional design, as well as the number of adverse events, the scope of data analysis was limited. The authors suggest that future studies with a prospective design should be carried out to allow a better analysis of the associations found in this study.
ConclusionAccording to this study, most donors are male, married, with a mean age of 40 years and a mean number of 13.4 donations by plateletpheresis. This profile indicates loyalty on the part of donors, who are willing to perform an invasive procedure as an example of altruism and solidarity with others.
The frequency of adverse events in this type of donation is low; in this study it was 4.4%. This fact is important for the recruitment of new donors, as it is essential to guarantee safety to donors.
Obtaining data on the incidence of adverse events enables the dynamic review of the medical and nursing teams to improve safety and comfort for the donor, to minimize underreporting of these events, and to discuss and create a national system of hemovigilance for adverse events in donations.
It is essential that the multidisciplinary teams that directly assist donors in blood component donation centers know how to advise patients during the procedure, and educate them about the possibility of adverse events.
Because this is a relatively new procedure, the national literature does not provide much information on the subject. The number of adverse reactions identified in this study was low, and did not lead to more significant and consistent associations and conclusions. Nevertheless, the authors expect that this study may lead to the development and update of national studies regarding platelet donations by apheresis and the possible adverse effects.
Conflicts of interestThe authors declare no conflicts of interest.