The aim of this work was to evaluate the lymphocyte immunophenotype in an elderly population.
MethodsThis study enrolled 35 over 60-year-old volunteers and a control group composed of 35 young adults. The study included elderly without diseases that might affect the functioning of the immune system. These individuals were consulted by doctors and after a physical examination, laboratory tests were performed using a Beckman Coulter® flow cytometer. The GraphPad Prism computer program was employed for statistical analysis with the level of significance being set for p-values <0.05.
ResultsThere is a statistically significant reduction in the number of lymphocytes (CD8+, CD2+ and CD3+ cells) in the elderly compared to young adults. These low rates are explained by changes attributed to aging and may be partly responsible for the reduction in the cellular immune response, lower proliferative activity and the low cytotoxicity of lymphocytes.
ConclusionThese parameters showed greater impairment of adaptive immunity in the elderly population and can therefore explain the greater fragility of the aged body to developing diseases.
The immune system is our first line of defense against foreign agents. These invaders, viruses, bacteria and fungi, can be moderately aggressive, such as those responsible for the common cold or more harmful such as in meningitis or tuberculosis. However, this system has several internal control mechanisms that prevent the development of infections. The defense mechanisms range from mechanical protection to complex cellular and molecular mechanisms.1
Innate immunity acts as a first line of organism defense. The innate immune system consists of several components. First, the epithelial barrier prevents infections and if this defense is destroyed, a group of phagocytic cells are activated, including monocytes, macrophages and neutrophils.2
In addition, the immune system can elicit another type of response called the adaptive immune system, which is also effective but slower and longer lasting. Its main characteristics are immune memory and specificity. In acquired immune responses, lymphocytes, classified into two basic categories as T lymphocytes and B lymphocytes, act to destroy pathogens.3
With aging, the elderly are affected more by infections and are more likely to develop cancer than younger individuals. These clinical problems are attributed, at least in part, to the aging of the immune system, immunosenescence, which is associated with an imbalance in the immune function.4
One theoretical basis to explain the aging process is that the immune activity, similar to most other physiological functions, declines with age.5 Conversely, studies have shown that there is an increase of natural killer (NK) cells with increasing age. The real cause of this increase remains unknown, but it is believed that this compensates for the decline in T-cell response.6
Neutrophils, important cells of innate immunity, do not respond efficiently in the mobilization process of the elderly when the hematopoietic system is under stress, for example, during chemotherapy and in severe prolonged infections. In these circumstances, the process of migration of neutrophils from the bone marrow into the systemic circulation is not as active as in youth people.7 Moreover, studies with different age groups demonstrated a significant decline in the phagocytic ability of neutrophils against bacteria as well as in the number of bacteria engulfed with advancing age.8
Research has shown that aging is accompanied by progressive changes in the composition of lymphocyte subsets (CD4 and CD8) in lymphoid tissues, with changes associated to T-cell function including those in relation to cytokine secretion.6
One of the major changes in the immune response in respect to the senescence system is the reduction of virgin T cells and increase in memory clones, in other words, CD4+ and CD8+ memory T cells expand while CD4+ and CD8+ virgin T cells gradually decrease with aging.9
An evaluation of the immune system may help to develop interventions in the elderly to avoid health problems that would eventually affect the independence of these individuals. Thus, early treatment may revert damage that under other circumstances would become irreversible.10
ObjectiveThe aim of this study was to evaluate the profile of the normal lymphocyte immunophenotypes in a population of healthy elderly people.
MethodsThis research is a descriptive study of lymphocyte immunophenotyping in an elderly population. Therefore, participants should be at least 60 years old and as healthy as possible.
Interviewers visited more than 200 homes to ascertain whether any of the residents were over 60 years old. They explained the purpose of the research and invited those who were interested to participate in the study.
The study group was selected by the Geriatrics Department, University Hospital Walter Cantídio (UHWC) of the Universidade Federal do Ceará (UFCE). For the physical evaluation, physicians requested basic biochemical tests, including complete blood count, total cholesterol, triglycerides, blood sugar, and creatinine in order to better assess each individual. After this evaluation, the study enrolled 35 of 200 elderly, who were physically healthy and had normal results for all these exams.
Informed consent was obtained from all participants and the study was approved by the Research Ethics Committee of the UFCE.
Thus, the population consisted of 61- to 92-year-old individuals of both genders and without chronic diseases, such as rheumatoid arthritis and systemic lupus erythematosus. Moreover the participants did not have acute infections such as colds and viruses, mental disability or had been diagnosed with depressive syndrome.
Individuals with autoimmune diseases, or other problems that may impair the immune system and those suffering from renal failure, severe heart disease or anemia were excluded from the study. Other exclusion criteria were the use of corticosteroids or immunosuppressive medications.
A control group of randomly chosen young adults was also created mainly of companions of patients who were waiting to be consulted at HUWC. Controls were submitted to the same tests as the study group and only those with normal results were enrolled in the study.
Blood samples were collected by venipuncture using sterile disposable equipment in a tube with 5mL of ethylenediaminetetraacetic acid (EDTA) as anticoagulant. This sample was used to perform immunophenotyping (markers of lymphocytes) with all tests being carried out on the same day the blood was drawn to guarantee the results.
The investigation of the immunophenotype was made using a Beckman Coulter® flow cytometer to obtain counts and percentages of the subpopulations of lymphocytes.
Following the manufacturer's instructions, 100mL of whole blood were incubated for 15minutes with a combination of labeled monoclonal antibodies (10mL each) in the dark at room temperature. Monoclonal antibodies, of the same brand as the flow cytometer, were conjugated with specific fluorochromes. After incubation, the samples were subjected to lysis using a standard reagent (OptiLyse® C).
Cells with the different markers (CD3, CD4, CD8, CD2, CD19) were counted using a technique that has been described previously11. Data are reported as absolute numbers and percentages.
The GraphPad Prism computer program (version 5.0) was used for statistical analysis. The Mann–Whitney test was employed for nonparametric values and Student's t-test for parametric values, with the level of statistical significance for all tests being set for p-values <0.05.
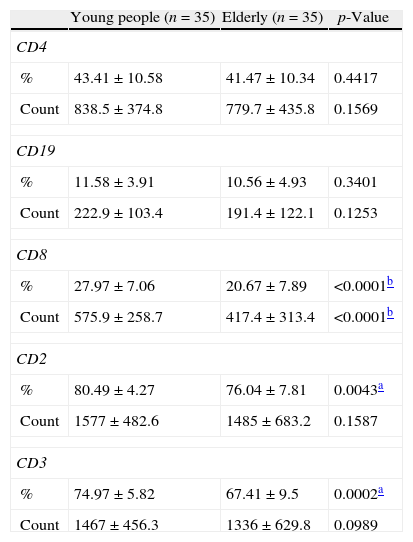
ResultsTables 1 and 2 show the absolute and relative numbers (percentages) and other data of lymphocytes in the Study and Control Groups.
Lymphocytes analyzed by flow cytometry.
| Young people (n=35) | Elderly (n=35) | p-Value | |
| CD4 | |||
| % | 43.41±10.58 | 41.47±10.34 | 0.4417 |
| Count | 838.5±374.8 | 779.7±435.8 | 0.1569 |
| CD19 | |||
| % | 11.58±3.91 | 10.56±4.93 | 0.3401 |
| Count | 222.9±103.4 | 191.4±122.1 | 0.1253 |
| CD8 | |||
| % | 27.97±7.06 | 20.67±7.89 | <0.0001b |
| Count | 575.9±258.7 | 417.4±313.4 | <0.0001b |
| CD2 | |||
| % | 80.49±4.27 | 76.04±7.81 | 0.0043a |
| Count | 1577±482.6 | 1485±683.2 | 0.1587 |
| CD3 | |||
| % | 74.97±5.82 | 67.41±9.5 | 0.0002a |
| Count | 1467±456.3 | 1336±629.8 | 0.0989 |
Counts in cells/mm3.
Results are reported as means±standard deviation.
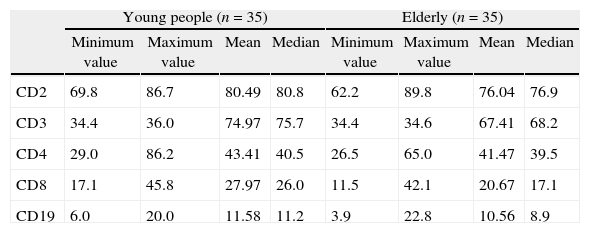
Values of the surface markers of lymphocytes.
| Young people (n=35) | Elderly (n=35) | |||||||
| Minimum value | Maximum value | Mean | Median | Minimum value | Maximum value | Mean | Median | |
| CD2 | 69.8 | 86.7 | 80.49 | 80.8 | 62.2 | 89.8 | 76.04 | 76.9 |
| CD3 | 34.4 | 36.0 | 74.97 | 75.7 | 34.4 | 34.6 | 67.41 | 68.2 |
| CD4 | 29.0 | 86.2 | 43.41 | 40.5 | 26.5 | 65.0 | 41.47 | 39.5 |
| CD8 | 17.1 | 45.8 | 27.97 | 26.0 | 11.5 | 42.1 | 20.67 | 17.1 |
| CD19 | 6.0 | 20.0 | 11.58 | 11.2 | 3.9 | 22.8 | 10.56 | 8.9 |
Values are shown as percentages.
The percentages of the lymphocytes with the CD8+, CD2+, and CD3+ markers were significantly lower in the group of elderly than in the young adult control group. Moreover the absolute number of lymphocytes with the CD8+ marker was significantly lower in the elderly than in young adults.
DiscussionThe present study evaluated the immunophenotype of lymphocytes to identify possible changes that accompany aging. A decrease in the relative numbers of cells expressing the CD2+ and CD3+ markers were observed in the elderly compared to young individuals. Moreover, decreases in both relative and absolute numbers were seen for lymphocytes with the CD8+ marker.
The absolute values (cells/mm3) are calculated indirectly from the value obtained for the total lymphocyte count and the percentage for each of the different subpopulations. It is known that absolute values of subpopulations characterize the biological condition of each individual better than the total lymphocyte count. It is also known that the absolute values of lymphocyte subpopulations characterize the biological condition of each individual better than the relative values (percentages) and so these are used in the clinical practice.
The work of Mazari and Lesourd in 1998, which evaluated 11 healthy elderly people, found a mean count of 1957cells/mm3 and 91.5% for CD2+ lymphocytes.12 In this study, the mean count was 1485cells/mm3 and 76.04% for the elderly group. This difference may be due to several factors, for example, the monoclonal antibodies used or because the population samples are from different regions.
We found no studies that show statistically significant differences for the percentages of the different subpopulations of lymphocytes in elderly subjects compared to young adults.
A study of the elderly by Sansoni et al.13 in 2008 evaluated lymphocyte subpopulations and found a drop in the absolute number of CD3+, CD4+ and CD8+ cells. In relation to our study, lower values in the absolute number of lymphocytes were found only in the subpopulation of CD8+ cells. Perhaps, if the study had a larger study sample a statistically significant difference may also have been found for CD3+ cells as the p-value (0.0989) was close to the cut-off of 0.05.
Lesourd and Conde-Martins14 reported that a drop in CD8+ lymphocytes, even though modest, is significantly more important than a drop in CD4+ cells.
ConclusionA decrease of immune cells was observed in healthy elderly people compared with young adults, especially in relation to lymphocytes with the CD8 phenotype. Thus, the lower numbers of lymphocytes in the elderly may affect the body's defenses and simple illnesses such as viruses may have serious consequences in this population. These values might serve as a reference for the normal aged population in our region.
These results show greater impairment of adaptive immunity in the elderly and may well justify a greater fragility of the elderly to diseases such as cancer.
Conflicts of interestThe authors declare no conflicts of interest.