Leukemia cutis is a rare entity diagnosed in only 1–3% of T- and B-cell acute lymphoblastic leukemia (ALL).1,2 Aleukemic leukemia cutis (ALC) is an even rarer diagnosis, occurring without leukemic cells in the blood or marrow, often preceding systemic disease.2,3 The Philadelphia chromosome (Ph) is one of the most common chromosomal abnormalities in adult B-ALL patients and is associated with poor prognosis. Although the use of tyrosine kinase inhibitors (TKIs) targeting the oncoprotein breakpoint cluster region-Abelson murine leukemia 1 (BCR-ABL1) has dramatically improved outcomes, allogeneic hematopoietic stem cell transplant (HSCT) is still recommended for all eligible patients, with relapse after HSCT remaining a major cause of treatment failure.4 Herein we report a case of isolated skin relapse of Ph-positive pre-B cell ALL after allogeneic HSCT.
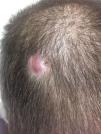
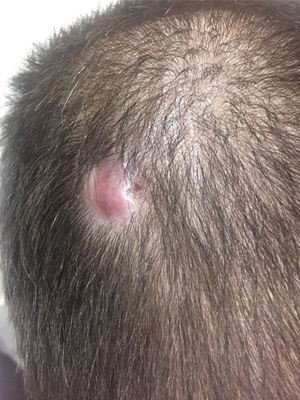
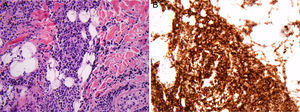
Case reportA 26-year-old man received a matched related donor peripheral blood HSCT for Ph-positive pre-B cell ALL in first remission. Prior to HSCT he had achieved complete molecular remission after two cycles of imatinib and the regimen rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine (R-HyperCVAD). Remission was consolidated with an allogeneic transplant from his human leukocyte antigen (HLA)-matched sibling using cyclophosphamide and 12Gy total body irradiation conditioning. Graft-versus-host disease (GvHD) prophylaxis consisted of tacrolimus and methotrexate. His post-HSCT course was complicated by chronic GvHD involving the lungs, liver, skin and lacrimal glands; he was treated with extracorporeal photopheresis, tacrolimus and prednisone. An isolated 2-cm erythematous and elevated scalp skin nodule was noticed 15 months after transplant (Figure 1). Biopsy showed atypical monomorphous mononuclear cells infiltrating the dermis and subcutaneous fat (Figure 2A); the cells stained positive for CD19 (Figure 2B), CD34, TDT, CD10, and PAX5 by immunohistochemistry, consistent with ALL. There was no evidence of systemic disease in exams of the bone marrow, peripheral blood or cerebrospinal fluid, by flow cytometry, cytogenetics, or in an investigation of the BCR-ABL1 gene by fluorescent in situ hybridization (FISH) and quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), or by whole-body positron emission tomography (PET) scan and brain magnetic resonance imaging. Stable engraftment with full donor chimerism was observed. Next generation sequencing of biopsy tissue targeting a large panel of known hematologic malignancy mutations showed the BCR-ABL1 fusion gene and additional genomic alterations of unknown significance (FoundationOne Heme, Cambridge, MA).5 Skin FISH testing also showed the BCR-ABL1 fusion. Treatment consisted of local radiation (24Gy), followed by dasatinib, an inhibitor of the Abl, Src and c-Kit kinases. The patient remains in remission 25 months after HSCT, while on chronic GvHD treatment.
Discussion and conclusionTo the best of our knowledge, this is the first report of Ph-positive ALL isolated skin relapse after an allogeneic HSCT. A MEDLINE/PubMed search with the terms “aleukemic leukemia cutis” AND “lymphoblastic leukemia” yielded eight results. Of these, only one case described ALC relapse of B-ALL after allogeneic transplantation.3 Remarkably, our patient has no detectable systemic disease despite the availability of sensitive molecular tests. In addition, the translocation (9;22) and the BCR-ABL1 gene were detected only in the skin, allowing targeted treatment of the relapse. Long-term prognosis of ALC recurrence after allogeneic HSCT and the potential benefit of tyrosine-kinase inhibitor therapy in addition to the graft-versus-leukemia effects are unknown.
Ethical statementThis protocol was approved by the Institutional Review Board of University Hospitals Cleveland Medical Center, and the subject gave written informed consent.
Conflicts of interestThe authors declare no conflicts of interest.