Alpha-thalassemia is a hereditary disease with a worldwide distribution characterized by reduced or absent synthesis of hemoglobin ¿ chains. Deletions involving the ¿ globin genes, which are duplicated (¿2 and ¿1) and located in the ¿ cluster (16p13.3), are the most common causes of the disease and account for over 80% of cases. Loss of a functional ¿ gene in the haploid genome results in ¿+-thalassemia, which can occur in a heterozygous (-¿/¿¿) or homozygous(-¿/-¿) state, while loss of both ¿ genes results in ¿0-thalassemia,which can also occur in a heterozygous (--/¿¿) or homozygous (--/--) state. A fifth ¿-thalassemic genotype is the result of the combination of both the ¿0 and ¿+ alleles (-¿/--). While the first three genotypes are associated with minimal hematological changes and the fourth results in hemoglobin (Hb) Bart's hydrops fetalis with intrauterine or neonatal death, the double heterozygous ¿0/¿+ (-¿/--) state leads to Hb H disease. This latter is characterized by unstable ¿ chain tetramers (¿4), causing chronic, moderate to severe hemolytic anemia with microcytosis, hypochromia, jaundice and hepatosplenomegaly.1,2
There are seven deletions that usually affect populations around the world: [-¿3.7, -¿4.2, -(¿)20.5, --MED, --SEA, --FIL, --THAI]. The most common method used to screen for these deletions is multiplex-gap polymerase chain reaction (PCR).3 When the molecular basis of the disease cannot be identified in this way, multiplex ligation-dependent probe amplification (MLPA) can be used to detect new or rare deletions in the ¿ genes, in the whole cluster and in the ¿-major regulatory element (MRE) located 40kb downstream of the ¿ gene.1,2
We describe the case of a Brazilian patient with Hb H disease caused by the combination of the -¿3.7 deletion, the most common cause of ¿-thalassemia in populations, and a rarer ¿0 deletion identified only by MPLA.
Case reportThis case study was part of a project approved by the Research Ethics Committee of the Universidade Estadual de Campinas (Unicamp) under reference number 918/2007.
A 31-year-old white Brazilian male of Italian descent from Araraquara, in the state of São Paulo, with a diagnosis of hypochromic microcytic anemia was referred to our laboratory to investigate the cause of his anemia. Cell counts and hematological indices were determined using an automated hematology analyzer (Sysmex XE5000, Sysmex, Japan) and hemoglobin analysis was carried out by electrophoresis on cellulose acetate in alkaline and neutral pHs and by cation-exchange high-performance liquid chromatography (HPLC) (Variant II™, Bio-Rad Laboratories, Hercules, CA, USA). In addition to the hemoglobin (Hb) A and Hb A2 fractions, an Hb H fraction was detected accounting for 4% of the total Hb. The patient's parents were analyzed, and although they did not have any clinical complaints, both had minor hematologic changes similar to those found in ¿-thalassemia.
The first molecular analysis consisted of multiplex-gap PCR,3 which showed a pattern in the patient's sample observed when the ¿3.7 deletion is in a homozygous state, a result that would not explain the Hb H disease. The samples were then analyzed by MLPA using the SALSA MLPA P140 C1 HBA kit (MRC Holland, Holland),4 which analyzes approximately 360kb of DNA extending from the 16p telomeric region to the DECR2 gene. The fragments were compared in Coffalyser.Net to identify possible changes in the number of copies of the ¿ alleles.
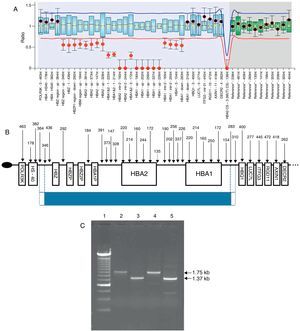
In addition to the -¿3.7 deletion, MLPA detected a large deletion of approximately 30kb affecting the ¿, ¿¿, ¿¿2, ¿¿1, ¿2 and ¿1 genes (Figure 1A). The extent of this deletion and the genes affected are compatible with two previously described deletions: --DUTCH, described in individuals of Dutch origin,5 and --MEDII, described in individuals of Mediterranean origin.2,6 These can be distinguished from each other by a specific PCR protocol.5 Here, the patient's DNA was first amplified with primers far from the deletion breakpoints, and the product was re-amplified by nested PCR with primers flanking the breakpoints of both deletions. With the --MEDII deletion, fragments of approximately 1.35 and 1.75kb are formed (Figure 1C), while with the --DUTCH deletion, the fragments are 1.03 and 1.41kb in size. Our results indicate that the deletion in question consists of the --MEDII deletion (Figure 1B) in combination with the -¿3.7 deletion (-¿3.7/--MEDII) in the patient in combination with the normal allele (--MEDII/¿¿) in the patient's father. The patient's mother was heterozygous for the -¿3.7 deletion (-¿3.7/¿¿). The hematological and molecular data for the family are shown in Table 1.
(A) Graph showing the result for the proband generated by the Coffalyser.Net software. The x-axis represents the probes and the y-axis the ratio of the intensity of the proband sample to the mean intensity of reference samples. A ratio of 1 indicates the presence of both alleles, a ratio of 0.5 the loss of one allele and 0 the loss of that region in both alleles. (B) Schematic representation of chromosome 16p13.3. The oval represents the telomeric region, the arrows show the locations of the probes and the boxes the genes. The blue line corresponds to the deleted fragment, the dotted lines denote the first and last deleted probe and the regions between the dotted and dashed lines show where the breakpoints may be (adapted from MRC-Holland, 2014). (C) Agarose gel with the nested-PCR amplified product. The 1.75kb and 1.35kb bands correspond to the --MEDII deletion.5 Sample 1 is the molecular weight marker (240bp ladder); samples 2 and 3 are from the proband, and samples 4 and 5 from his father.
Hematological data for the family studied.
| Family member | Proband | Father | Mother |
|---|---|---|---|
| RBC (RV – M: 4.5–6.1; F: 4.2–5.4) | 5.55 | 6.39 | 4.74 |
| Hb (RV – M: 14–18; F: 12–16) | 9.2 | 13.8 | 12.5 |
| Ht (%) (RV – M: 41–52; F: 36–46) | 16.6 | 44.0 | 38.6 |
| MCV (RV: 80–99) | 61.6 | 81.4 | 68.9 |
| MCH (RV: 27–32) | 16.6 | 21.6 | 26.4 |
| RDW (%) (RV: 10–15) | 25.5 | 14.5 | 15.3 |
| RC (%) (RV: 0.5–2.5) | 2.77 | 1.49 | 0.98 |
| Hb pattern | A2, A, H | A2, A | A2, A |
| Hb A2 (%) (RV: 1.6–4) | 1.5 | 2.70 | 2.40 |
| Hb F (%) (RV: <2) | 0.5 | 0.20 | 0.20 |
| Hb H (%) | 4.0 | - | - |
| Heinz bodies/Hb H bodies | Positive | Negative | Negative |
| ¿ genotype | -¿3.7/--MEDII | --MEDII/¿¿ | -¿3.7/¿¿ |
RV: reference values; RBC: red blood cell count (×109/¿L); Hb: hemoglobin (g/dL); Ht: hematocrit (%); MCV: mean corpuscular volume (fL); MCH: mean corpuscular hemoglobin (pg); RDW: red cell distribution width (%); RC: reticulocyte count (%).
Alpha-thalassemias are frequently caused by deletions. The most common of these is the -¿3.7 deletion, which has a prevalence of 20–25% in Afro-Brazilians.7,8 Hb H disease is sporadic in Brazil and is generally caused by a combination of the -¿3.7 deletion and --MEDI, --SEA or -(¿)20.5 deletions.9 MLPA, however, has made it possible to detect rarer and even novel ¿0 deletions and even those that only affect the regulatory element. In the family analyzed here, Hb H disease was the result of a combination of the -¿3.7 allele and --MEDII deletion, a genetic alteration not previously reported in the Brazilian or Latin American population. This deletion is larger than the --MEDI (which is approximately 17kb of DNA) and removes the zeta gene in addition to the alpha genes. It has been found in Mediterranean countries, such as Italy, Turkey, Greece and Cyprus.6,10
Here, molecular analysis using MLPA, confirmed by familial analysis, played an important role in the diagnosis. MLPA has made it possible to detect a wide range of mutations affecting the globin genes in the Brazilian population that are not detected by other techniques.
Financial supportThis study was carried out with financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grant no. 2014/00984-3; fellowship no. 2015/21184-8), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) of the Brazilian Ministry of Education.
Conflicts of interestThe authors declare no conflicts of interest.