Cyclophosphamide plus thalidomide as induction for multiple myeloma patients eligible for autologous stem cell transplantation may be a limiting factor for cell mobilization. The minimum acceptable mobilized peripheral blood stem cell count to prevent deleterious effects during transplantation is 2.0×106 CD34+ cells/kg. Combining other treatments to granulocyte-colony stimulating factor, such as cyclophosphamide, could overcome the mobilization limitation. The objective of this study was to assess the number of CD34+ cells mobilized using granulocyte-colony stimulating factor with and without cyclophosphamide after induction with cyclophosphamide, thalidomide and dexamethasone.
MethodsA retrospective study was performed of a cohort of multiple myeloma patients submitted to autologous stem cell transplantations at two Brazilian centers between May 2009 and July 2013. The oral cyclophosphamide and thalidomide induction doses used were 1500mg/month and 100–200mg/day, respectively. Mobilization doses were 10–15mcg/kg granulocyte-colony stimulating factor with 2–4g/m2 cyclophosphamide, or 15–20mcg/kg granulocyte-colony stimulating factor alone for 5 days. Collection of >2.0×106 CD34+ cells/kg was considered sufficient.
ResultsEighty-eight patients were analyzed; only 18 received cyclophosphamide. The median age was 58 years old (range: 51–62) for the granulocyte-colony stimulating factor group and 56.5 years old (range: 54–60) for granulocyte-colony stimulating factor plus cyclophosphamide group. Fifty-two patients were male. Eighty cases (90.9%) were Durie-Salmon Staging System III-A/B and 38 (44.7%) and 20 cases (23.5%) were International Staging System 2 and 3, respectively. The group that received cyclophosphamide collected a higher median number of progenitor cells [3.8 (range: 3.1–4.4) vs. 3.2 (range: 2.3–3.8)] (p-value=0.008). No correlation was observed between better responses or number of induction cycles and the number of cells collected.
ConclusionThe number of cells mobilized with granulocyte-colony stimulating factor plus cyclophosphamide was higher. However, in both groups, the median number of CD34+ cells was sufficient to perform a single autologous stem cell transplantation; no deleterious effects were reported during harvesting.
The use of high-dose chemotherapy plus autologous stem cell transplantation (ASCT) as consolidation after chemotherapy induction has been the first line of treatment for eligible multiple myeloma patients for over three decades.1 Currently, the most commonly employed induction strategy is to use a number of cycles (4–6) with three drugs.1 The introduction of triple combinations of novel agents for induction, such as the immunomodulators thalidomide and lenalidomide or the proteasome inhibitor bortezomib, has significantly changed as these drugs resulted in better outcomes and tolerability compared to classic regimens such as vincristine, doxorubicin and dexamethasone (VAD).1 However, the induction regimens should not affect hematopoietic progenitors in the mobilization process.2–6 Recently, this influence on mobilization has been gaining increased attention due to the use of chemotherapy combinations involving novel agents such as immunomodulators (lenalidomide) and, in particular, alkylating agents (cyclophosphamide) which can increase hematologic toxicity.2,6 Auner et al.2 found that the combination of cyclophosphamide, thalidomide and dexamethasone (CTD) in the induction of patients with multiple myeloma significantly reduced mobilization of progenitor cells compared to the classic VAD or VAD-like regimens, even when using cyclophosphamide in association with granulocyte colony-stimulating factor (G-CSF) during mobilization. In an effort to overcome this mobilization problem, other agents have been associated with G-CSF, most notably plerixafor.7 However, the high cost of plerixafor limits its use in many centers. Chemotherapy associated with G-CSF can significantly increase the mobilization of progenitor cells. One of the most used chemotherapy drugs in mobilization based on a combination with G-CSF is cyclophosphamide, administered at a typical dose of 2–4g/m2.8 However, this treatment has some drawbacks given that it raises the cost of the procedure owing to the need for hospitalization of patients and can lead to slower bone marrow engraftment, greater toxicity with pancytopenia, neutropenia, infections and death.9–12 The number of CD34+ collected for ASCT depends on several factors the most important of which are the number of transplantations planned and the least impact in terms of time on the mobilized peripheral blood stem cells. Traditionally, the target for CD34+ cell collection for single ASCT has been 4–6×106cells/kg, with the value also hinging on the deleterious impact of harvesting at counts of below 2×106 CD34+ cells/kg, defined as the lowest acceptable level.3 Greater numbers of CD34+ cells have not been associated with any significant benefit in the parameters studied.6 Another objective in the quantity of cells mobilized is to allow for a cell reserve for a second ASCT as rescue in the event of future disease relapse, rendering the target cell count in the first mobilization ≥4×106 CD34+ cells/kg.8,13 Some peculiarities exist in Brazil which hamper the use of ASCT such as low number of beds for transplantations and the shortage of frozen cell storage for second transplants within the Brazilian National Health System (SUS). The three-drug induction regimen widely used in Brazil for multiple myeloma patients is cyclophosphamide, thalidomide and dexamethasone (CTD).14 The primary objective of this study was to determine whether the collection of progenitor cells using G-CSF alone is sufficient to perform at least one ASCT, compared with a group undergoing mobilization with G-CSF associated with cyclophosphamide, in patients submitted to the CTD chemotherapy regimen for induction.
MethodsA retrospective, sequential analysis of 88 multiple myeloma patients who had been mobilized for peripheral blood stem cell harvest and submitted to ASCT at two Brazilian centers between May 2009 and June 2013 was conducted. All patients were induced using the CTD regimen. Center 1, Hospital da Irmandade da Santa Casa de Misericórdia de São Paulo, used a continuous infusion of cyclophosphamide (50mg/day), a continuous infusion of thalidomide (100–200mg/day) and dexamethasone (160mg/month). Center 2, Hospital das Clinicas of the Faculdade de Medicina da Universidade de SP (HC-FMUSP), used cyclophosphamide (500mg) on Days 1, 8 and 15, thalidomide (100mg/day) and dexamethasone (200–400mg/month). All the agents were administered orally with four to six planned induction cycles. The protocol used for mobilization differed between the centers giving two groups for comparison: Group 1 received cyclophosphamide (2–4g/m2 split in 2 doses) associated with G-CSF (10–15mcg/kg until the collection of cells), and Group 2 received G-CSF (15–20mcg/kg for 5 days) alone. All patients were submitted to an outpatient mobilization protocol. The CD34+ cell count for collection was determined by flow cytometry using a FACSCalibur BD device with double platform and employing the cell quest program and ISHAGE protocol. Apheresis was performed according to the CD34+ count in peripheral blood (≥10×103/mL) starting between Day 7 and 10 in Group 1 and Day 4 and 10 in Group 2, with the median day of collection being Days 7 and 4 after commencing G-CSF for Groups 1 and 2, respectively. The patients were submitted to a large volume leukapheresis protocol with a median of four blood volume apheresis (range: 3–6). The Cobe® Spectra Marc Terumo BCT apheresis system was used in both centers. CD34+ cell collection was considered adequate with a count ≥2.0×106 CD34+ cells/kg. As the CD34+ cells for the transplants had come from patients who had their cells collected previously, it was not possible to analyze data on failure of mobilization. Progression-free survival (PFS) and overall survival (OS) were analyzed. PFS was defined as the time elapsed between the start of induction treatment to disease progression or death, with censure on the date of last contact. OS was defined as the time elapsed between the start of induction treatment until death, with censure on the date of last contact. The unpaired t-test was used to compare means of variables with a normal distribution, whereas the Mann–Whitney test was employed to compare variables with a non-normal distribution. Categorical variables, including response rates, were compared using the Chi-square or Fisher exact tests, as applicable. Survival analyses were carried out using the Kaplan–Meier technique while the comparison between groups was performed with the log rank test. Median follow-up for OS was calculated using the reverse Kaplan–Meier method. Analyses were carried out using the MedCalc software (Mariakerke, Belgium, v. 11.3.3.0). Values with a two-tailed p-value <0.05 were considered statistically significant. The study protocol was approved by both institutions, with data collected from a database derived from the Grupo Brasileiro de Mieloma Múltiplo (GBRAM 003) study. This study was approved by the Research Ethics Committee of the Hospital da Irmandade da Santa Casa de Misericórdia de São Paulo and a consent form was waived given the retrospective nature of the study.
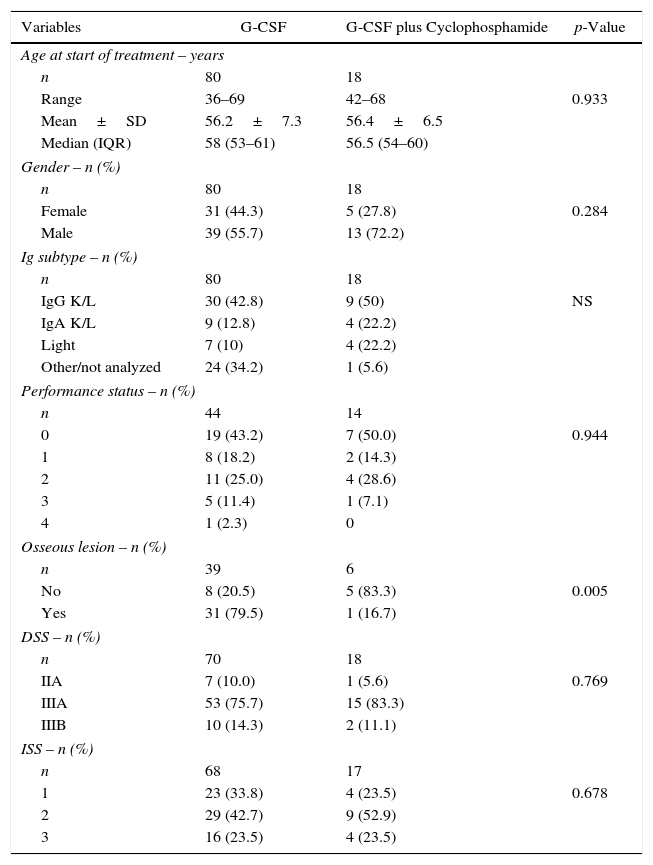
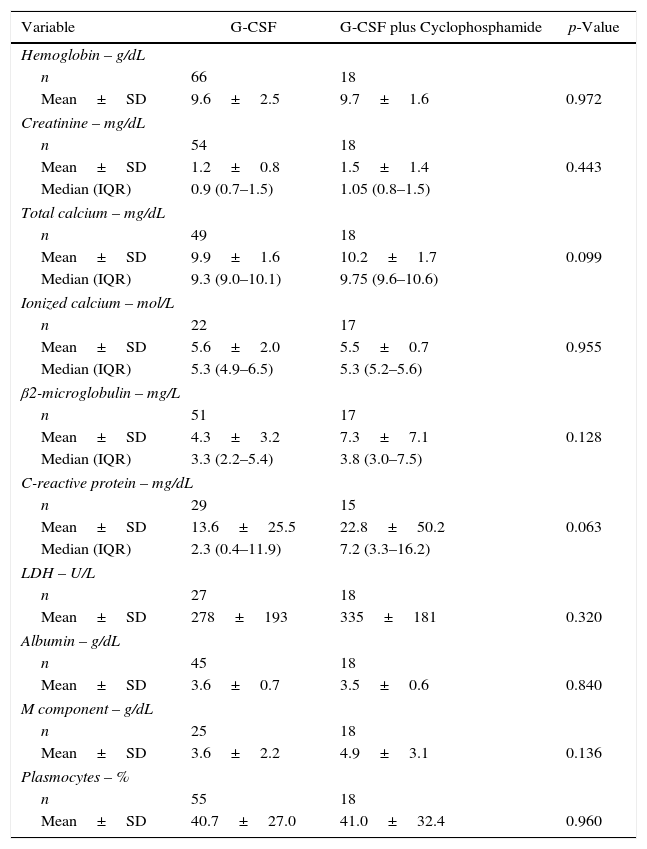
ResultsDemographic and baseline clinical characteristics of patientsA total of 88 patients with multiple myeloma submitted to ASCT after induction using CTD were included. Regarding the mobilization scheme, 70 patients received filgrastim alone, and 18 received a combination of filgrastim and cyclophosphamide. The main demographic and clinical characteristics of the 88 patients stratified by mobilization scheme are shown in Table 1. The groups did not differ in respect to age (median: 58; range: 51–62 years) or gender and the groups were balanced in terms of performance status or prognostic index using the Durie-Salmon Staging System (DSS) and International Staging System (ISS). Table 2 depicts the distribution of the main laboratory variables at the time of diagnosis. No significant difference was found between the groups for any of the variables studied. Given the retrospective nature of the study, there was a large amount of missing data for some variables (total calcium, ionized calcium, creatinine, lactate dehydrogenase (LDH), Beta-2 microglobulin) in the group submitted to mobilization using G-CSF alone.
Patient characteristics.
| Variables | G-CSF | G-CSF plus Cyclophosphamide | p-Value |
|---|---|---|---|
| Age at start of treatment – years | |||
| n | 80 | 18 | |
| Range | 36–69 | 42–68 | 0.933 |
| Mean±SD | 56.2±7.3 | 56.4±6.5 | |
| Median (IQR) | 58 (53–61) | 56.5 (54–60) | |
| Gender – n (%) | |||
| n | 80 | 18 | |
| Female | 31 (44.3) | 5 (27.8) | 0.284 |
| Male | 39 (55.7) | 13 (72.2) | |
| Ig subtype – n (%) | |||
| n | 80 | 18 | |
| IgG K/L | 30 (42.8) | 9 (50) | NS |
| IgA K/L | 9 (12.8) | 4 (22.2) | |
| Light | 7 (10) | 4 (22.2) | |
| Other/not analyzed | 24 (34.2) | 1 (5.6) | |
| Performance status – n (%) | |||
| n | 44 | 14 | |
| 0 | 19 (43.2) | 7 (50.0) | 0.944 |
| 1 | 8 (18.2) | 2 (14.3) | |
| 2 | 11 (25.0) | 4 (28.6) | |
| 3 | 5 (11.4) | 1 (7.1) | |
| 4 | 1 (2.3) | 0 | |
| Osseous lesion – n (%) | |||
| n | 39 | 6 | |
| No | 8 (20.5) | 5 (83.3) | 0.005 |
| Yes | 31 (79.5) | 1 (16.7) | |
| DSS – n (%) | |||
| n | 70 | 18 | |
| IIA | 7 (10.0) | 1 (5.6) | 0.769 |
| IIIA | 53 (75.7) | 15 (83.3) | |
| IIIB | 10 (14.3) | 2 (11.1) | |
| ISS – n (%) | |||
| n | 68 | 17 | |
| 1 | 23 (33.8) | 4 (23.5) | 0.678 |
| 2 | 29 (42.7) | 9 (52.9) | |
| 3 | 16 (23.5) | 4 (23.5) | |
G-CSF: granulocyte-colony stimulating factor; SD: standard deviation; IQR: interquartile range; DSS: Durie-Salmon Staging; ISS: International Staging System.
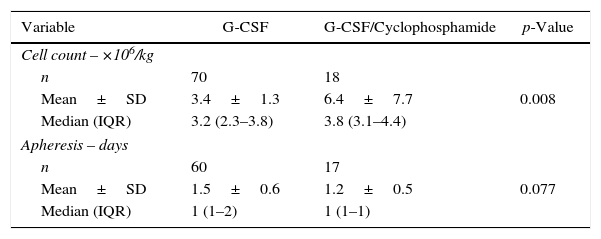
Laboratory variables.
| Variable | G-CSF | G-CSF plus Cyclophosphamide | p-Value |
|---|---|---|---|
| Hemoglobin – g/dL | |||
| n | 66 | 18 | |
| Mean±SD | 9.6±2.5 | 9.7±1.6 | 0.972 |
| Creatinine – mg/dL | |||
| n | 54 | 18 | |
| Mean±SD | 1.2±0.8 | 1.5±1.4 | 0.443 |
| Median (IQR) | 0.9 (0.7–1.5) | 1.05 (0.8–1.5) | |
| Total calcium – mg/dL | |||
| n | 49 | 18 | |
| Mean±SD | 9.9±1.6 | 10.2±1.7 | 0.099 |
| Median (IQR) | 9.3 (9.0–10.1) | 9.75 (9.6–10.6) | |
| Ionized calcium – mol/L | |||
| n | 22 | 17 | |
| Mean±SD | 5.6±2.0 | 5.5±0.7 | 0.955 |
| Median (IQR) | 5.3 (4.9–6.5) | 5.3 (5.2–5.6) | |
| β2-microglobulin – mg/L | |||
| n | 51 | 17 | |
| Mean±SD | 4.3±3.2 | 7.3±7.1 | 0.128 |
| Median (IQR) | 3.3 (2.2–5.4) | 3.8 (3.0–7.5) | |
| C-reactive protein – mg/dL | |||
| n | 29 | 15 | |
| Mean±SD | 13.6±25.5 | 22.8±50.2 | 0.063 |
| Median (IQR) | 2.3 (0.4–11.9) | 7.2 (3.3–16.2) | |
| LDH – U/L | |||
| n | 27 | 18 | |
| Mean±SD | 278±193 | 335±181 | 0.320 |
| Albumin – g/dL | |||
| n | 45 | 18 | |
| Mean±SD | 3.6±0.7 | 3.5±0.6 | 0.840 |
| M component – g/dL | |||
| n | 25 | 18 | |
| Mean±SD | 3.6±2.2 | 4.9±3.1 | 0.136 |
| Plasmocytes – % | |||
| n | 55 | 18 | |
| Mean±SD | 40.7±27.0 | 41.0±32.4 | 0.960 |
G-CSF: granulocyte-colony stimulating factor; LDH: lactic dehydrogenase; M component: monoclonal component.
The data on the number of CD34+ collected was available for all patients, whereas the number of days of apheresis required for collection was available for only 77 cases. The group receiving G-CSF alone harvested a mean of 3.4±1.3×106/kg and a median of 3.2×106/kg (range: 2.3–3.8×106/kg) of CD34+ cells. On the other hand, the group that received filgrastim combined with cyclophosphamide harvested a higher number of progenitor cells with a mean of 6.4±7.7×106/kg and median of 3.8×106/kg (range: 3.1–4.4×106/kg) (p-value=0.008). No significant difference in the number of days for collection was observed between the groups (p-value=0.077). A summary of the number of cells mobilized and days of apheresis for the two groups is shown in Table 3.
Quantity of mobilized cells and days of apheresis for the G-CSF and G-CSF plus cyclophosphamide mobilization regimens.
| Variable | G-CSF | G-CSF/Cyclophosphamide | p-Value |
|---|---|---|---|
| Cell count – ×106/kg | |||
| n | 70 | 18 | |
| Mean±SD | 3.4±1.3 | 6.4±7.7 | 0.008 |
| Median (IQR) | 3.2 (2.3–3.8) | 3.8 (3.1–4.4) | |
| Apheresis – days | |||
| n | 60 | 17 | |
| Mean±SD | 1.5±0.6 | 1.2±0.5 | 0.077 |
| Median (IQR) | 1 (1–2) | 1 (1–1) | |
G-CSF: granulocyte-colony stimulating factor.
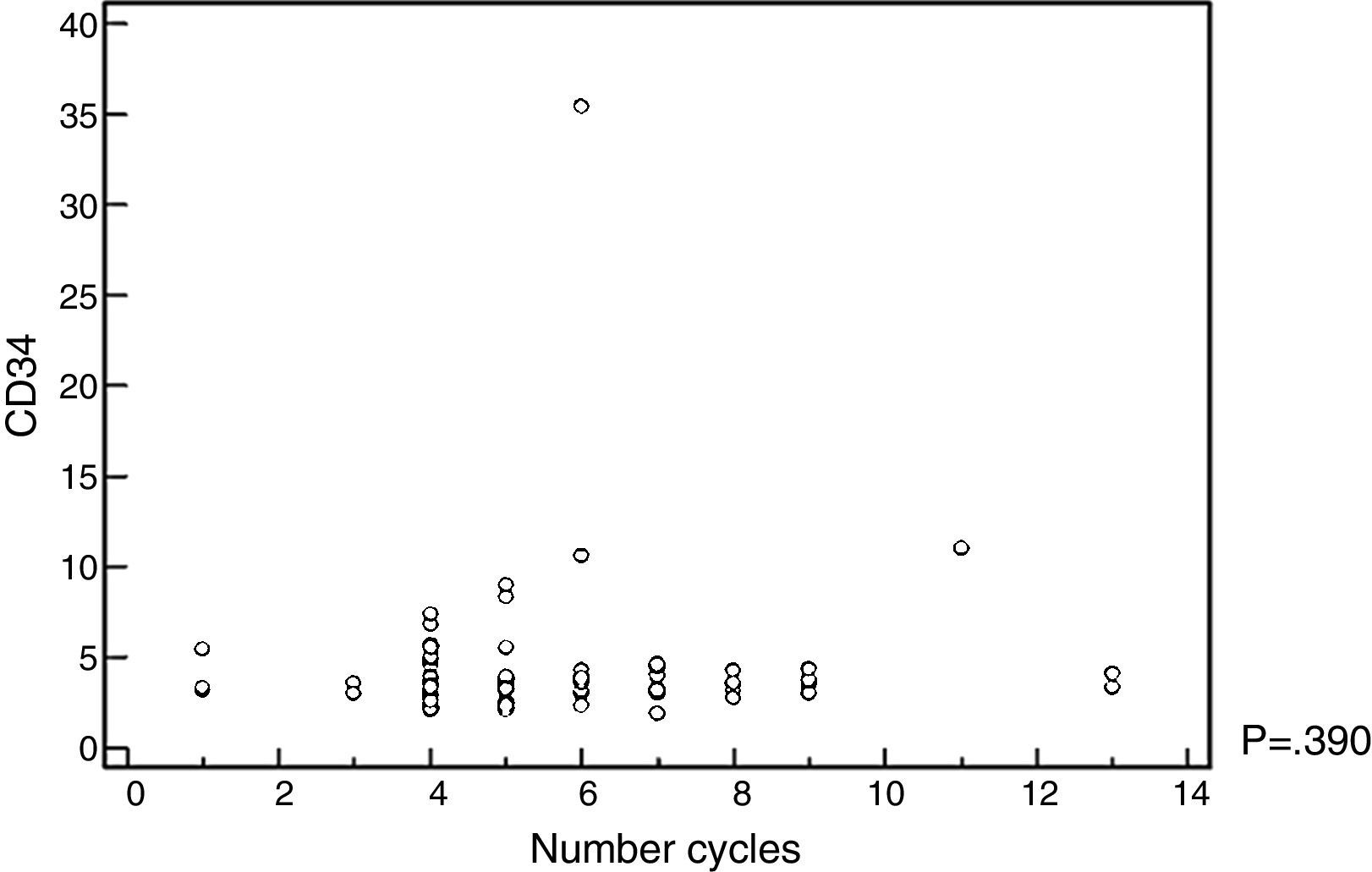
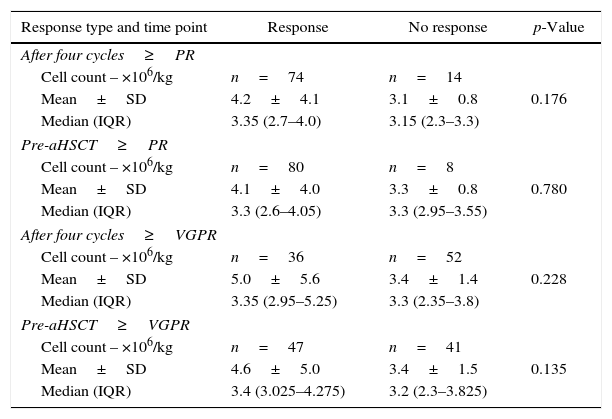
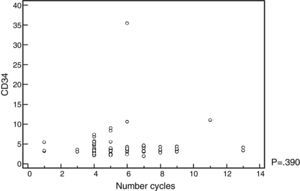
The mobilized CD34+-cell count was not influenced by the number of chemotherapy cycles administered, nor by the response rate prior to ASCT (Figure 1 and Table 4). No adverse events were recorded in the database register for the group that received cyclophosphamide associated with G-CSF.
Relationship between response type on induction and number of mobilized cells.
| Response type and time point | Response | No response | p-Value |
|---|---|---|---|
| After four cycles≥PR | |||
| Cell count – ×106/kg | n=74 | n=14 | |
| Mean±SD | 4.2±4.1 | 3.1±0.8 | 0.176 |
| Median (IQR) | 3.35 (2.7–4.0) | 3.15 (2.3–3.3) | |
| Pre-aHSCT≥PR | |||
| Cell count – ×106/kg | n=80 | n=8 | |
| Mean±SD | 4.1±4.0 | 3.3±0.8 | 0.780 |
| Median (IQR) | 3.3 (2.6–4.05) | 3.3 (2.95–3.55) | |
| After four cycles≥VGPR | |||
| Cell count – ×106/kg | n=36 | n=52 | |
| Mean±SD | 5.0±5.6 | 3.4±1.4 | 0.228 |
| Median (IQR) | 3.35 (2.95–5.25) | 3.3 (2.35–3.8) | |
| Pre-aHSCT≥VGPR | |||
| Cell count – ×106/kg | n=47 | n=41 | |
| Mean±SD | 4.6±5.0 | 3.4±1.5 | 0.135 |
| Median (IQR) | 3.4 (3.025–4.275) | 3.2 (2.3–3.825) | |
aHSCT: autologous hematopoietic stem cell transplantation; PR: partial response; VGPR: very good partial response.
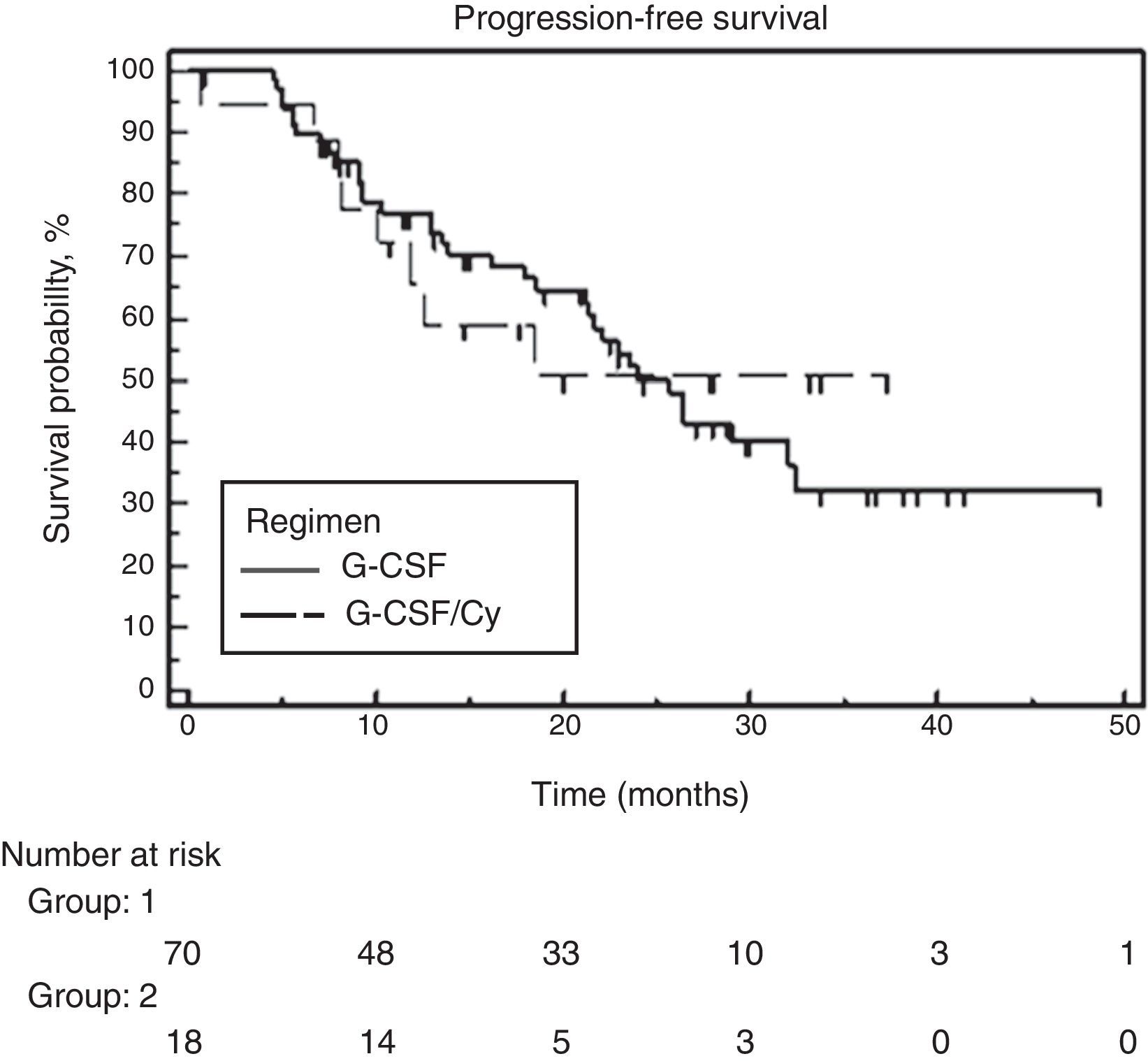
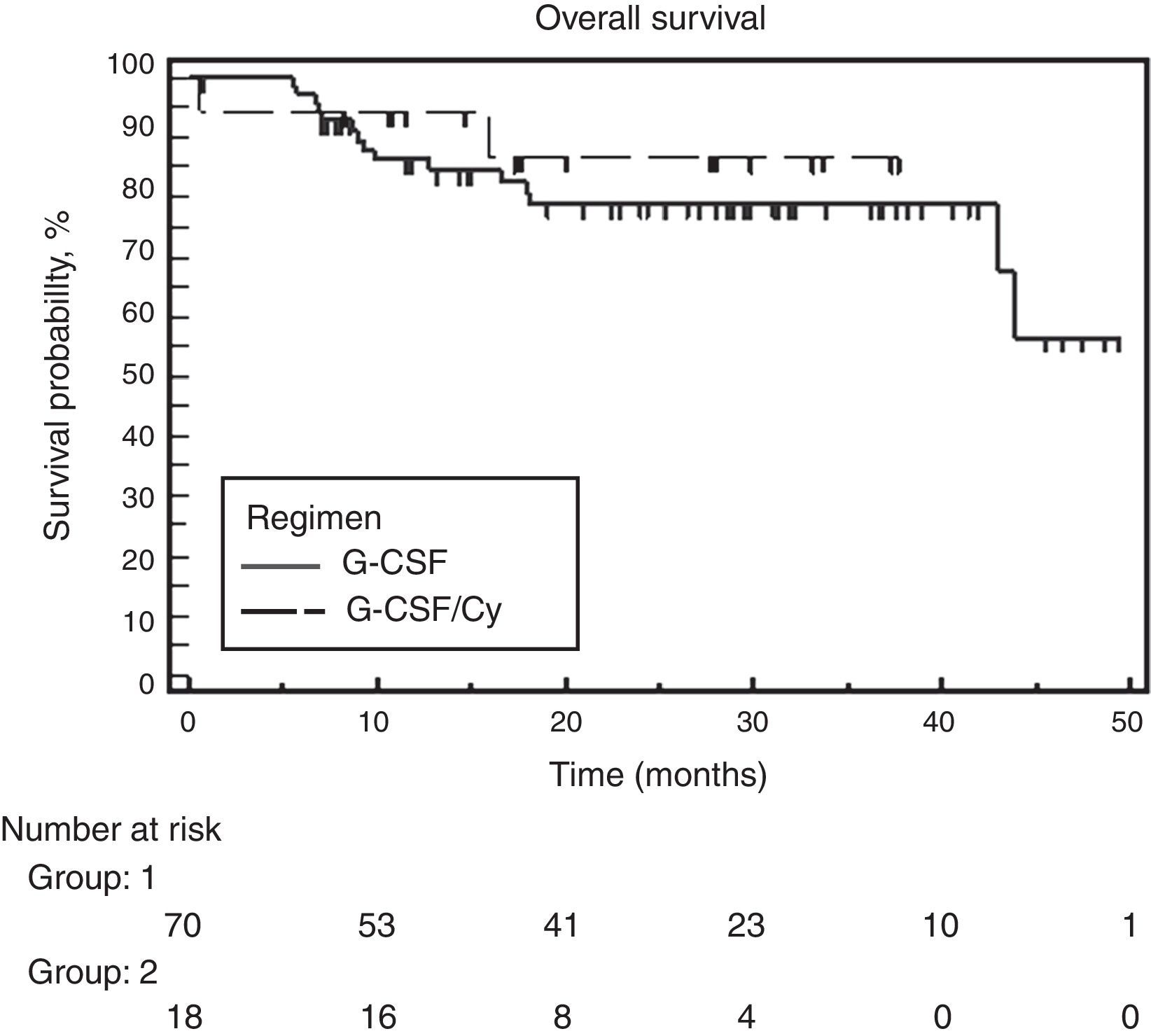
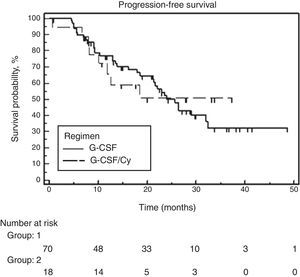
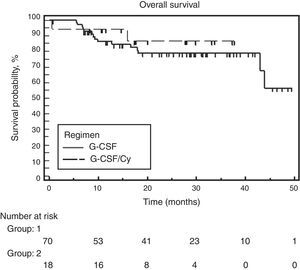
At the time of analysis, 39 of the 88 patients had suffered disease progression, and four had died. The median follow-up was 28.6 months and the overall PFS was 24 months. No significant difference was found between the two groups. Median PFS was 25.7 months for the G-CSF alone group and the PFS had not been attained for the group that received associated cyclophosphamide (Figure 2). Similarly, no difference was observed between the two groups for OS. Median OS had not been attained in either of the groups. The hazard ratio for OS was 0.65 (95% confidence interval: 0.18–2.27) favoring the cyclophosphamide regimen but not reaching significance (p-value=0.495) (Figure 3).
DiscussionThe aim of the present study was to analyze the quantity of mobilized progenitor cells with the use of G-CSF alone or in combination with the chemotherapy agent cyclophosphamide, in patients with newly diagnosed multiple myeloma submitted to chemotherapy induction using the CTD protocol. This is the first analysis of its kind published in the literature. The target quantity of mobilized cells differs between centers as some plan double ASCT, or allow for the freezing of some cells for a second transplant in the event of relapse while other centers plan collections for a single ASCT. Patients submitted to ASCT are generally over the age of 65 years where age ≥65 years has been identified as a predictive factor for poor mobilization.15,16 The median age of the patients in this study was 58 (range: 36–69) years, with no significant difference between the groups and therefore without predisposition to mobilization problems. Regarding clinical characteristics, there was a difference between the groups for the number of bone lesions, which was higher in the G-CSF group (p-value=0.005). More advanced disease stage has also been reported as a factor that negatively affects mobilization.16 No significant difference was found between the groups in relation to the DSS and ISS staging or performance status. Another factor identified as a risk factor for harvesting is the pre-mobilization tumor load.17 This load can be indirectly assessed based on the degree of response after induction. In the current study, there was no real advantage on comparing the quantity of mobilized cells for different response rates. The induction chemotherapy protocol for patients with multiple myeloma eligible for ASCT varies between centers worldwide in terms of type and combination of the agents employed. There is a strong, almost universal tendency for the use of novel agents such as bortezomib, lenalidomide and thalidomide, as combinations or with corticosteroids and/or alkylating agents.18–21 The use of a combination of three drugs in induction as opposed to two drugs yields greater benefits in response and survival.1 In Brazil, the only agent available for use in the public health system is thalidomide. The best combination identified and widely used in Brazilian centers is the CTD protocol. Currently, centers in developed countries prefer to use other drugs for induction however, combinations with thalidomide are still in use in Brazil and in other developing countries. This protocol was compared in a randomized phase III study against the VAD plus cyclophosphamide regimen in over 1000 patients with the CTD arm providing better response rates.20 The patients studied in the present investigation were selected from two national centers that use different protocols for the use of cyclophosphamide. However, the final monthly dose (1500mg) is the same in both: Center 1 uses a continuous daily oral dose of 50mg; Center 2 uses a dose of 500mg/week for three weeks every 28 days. In the only study published assessing the effect on mobilization in patients undergoing CTD, Auner et al.2 reported mobilization failure in attempts to perform at least one ASCT. Failure rates for cell number cut-offs of ≥4×106 CD34+ cells/kg and of ≥2×106 CD34+ cells/kg were 39% and 25% of the cases, respectively. In another study comparing CTD vs. a regimen of VAD plus cyclophosphamide as induction, Morgan et al. identified a mobilization failure rate of 1% of cases.19 In these studies, the combination of G-CSF and cyclophosphamide was used for mobilization in all cases. Transplant centers differ with regard to the standard conduct for harvesting progenitor cells. The use of cyclophosphamide, while promoting better cell collection, prolongs the whole process due to the wait for cell production recovery. Generally, this process is performed with the patient hospitalized and is associated with increased risk of febrile neutropenia and other infectious complications.6 For both of the centers in the present analysis, the minimum acceptable number of collected cells was 2×106 CD34+ cells/kg, thereby allowing for at least one ASCT to be carried out. A difference between groups was noted for the mobilization of progenitor cells, with the group that received G-CSF plus cyclophosphamide harvesting a higher mean of 6.4 (±7.7)×106/kg vs. 3.4 (±1.3)×106/kg for the group that received G-CSF alone (p-value=0.008). However, the number of cells in both groups was sufficient to perform at least one ASCT. Auner et al. reported a different number of days of apheresis in cases that received CTD vs. those induced using a VAD or VAD-like protocol. In the current study, no difference in the number of days of apheresis was observed between the groups (p-value=0.07). No studies exist that support an increase in induction time preceding mobilization to enhance successful cell collection.22 In the present study, no association was found between better response and improved survival. The cyclophosphamide used in mobilization, in addition to its ability to promote release of progenitor cells from the bone marrow for peripheral collection, has a reputation of reducing the disease further (debulking) during the collection of progenitor cells. Retrospective studies have failed to confirm this effect and have shown no advantage in terms of survival.22 Similarly, in the present study, no advantages of cyclophosphamide use were found in terms of mobilization, improved response or survival. Assessing the situation in the Brazilian context with regards to limitations for performing ASCT, difficulties in frozen storage of cells, and the need to cut costs, the present study revealed that sufficient progenitor cells can be mobilized to perform at least one ASCT with the use of G-CSF alone in patients induced using the CTD protocol.
ConclusionThe use of G-CSF alone to mobilize progenitor cells is feasible in multiple myeloma patients induced with a cyclophosphamide, thalidomide and dexamethasone protocol.
Conflicts of interestThe authors declare no conflicts of interest.