Anemia is a frequent complication in cancer patients, both at diagnosis and during treatment, with a multifactorial etiology in most cases. Iron deficiency is among the most common causes of anemia in this setting and can develop in nearly half of patients with solid tumors and hematologic malignancies. Surprisingly, this fact is usually neglected by the attending physician in a way that proper and prompt investigation of the iron status is either not performed or postponed. In cancer patients, functional iron deficiency is the predominant mechanism, in which iron availability is reduced due to disease or the therapy-related inflammatory process. Hence, serum ferritin is not reliable in detecting iron deficiency in this setting, whereas transferrin saturation seems more appropriate for this purpose. Besides, lack of bioavailable iron can be further worsened by the use of erythropoiesis stimulating agents that increase iron utilization in the bone marrow. Iron deficiency can cause anemia or worsen pre-existing anemia, leading to a decline in performance status and adherence to treatment, with possible implications in clinical outcome. Due to its frequency and importance, treatment of this condition is already recommended in many specialty guidelines and should be performed preferably with intravenous iron. The evidences regarding the efficacy of this treatment are solid, with response gain when combined with erythropoiesis stimulating agents and significant increments in hemoglobin as monotherapy. Among intravenous iron formulations, slow release preparations present more favorable pharmacological characteristics and efficacy in cancer patients.
Anemia is a frequent complication in cancer patients, both at diagnosis and during treatment. In the European Cancer Anaemia Survey (ECAS), 39% of patients were anemic at the time of enrollment in the study, and 67% had anemia during chemotherapy. The cause of anemia in these patients is multifactorial, and for many of them iron deficiency is the dominant mechanism.1,2
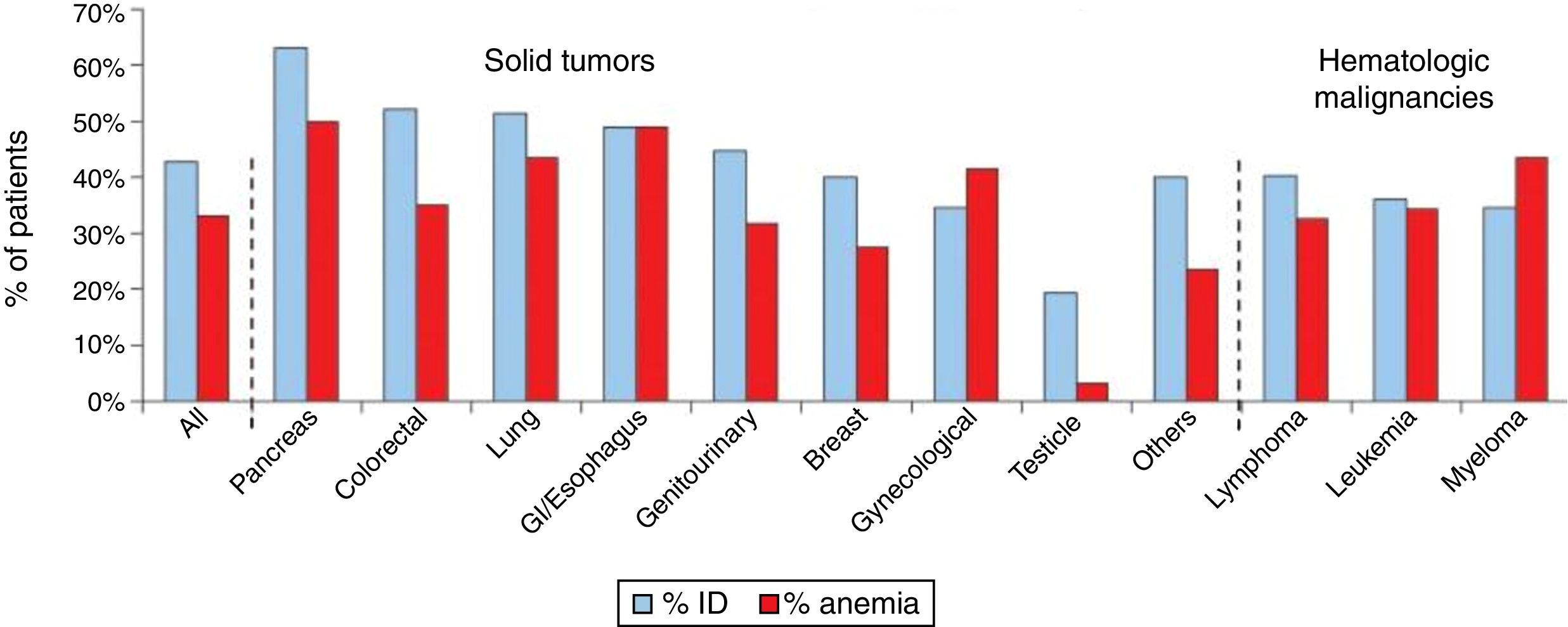
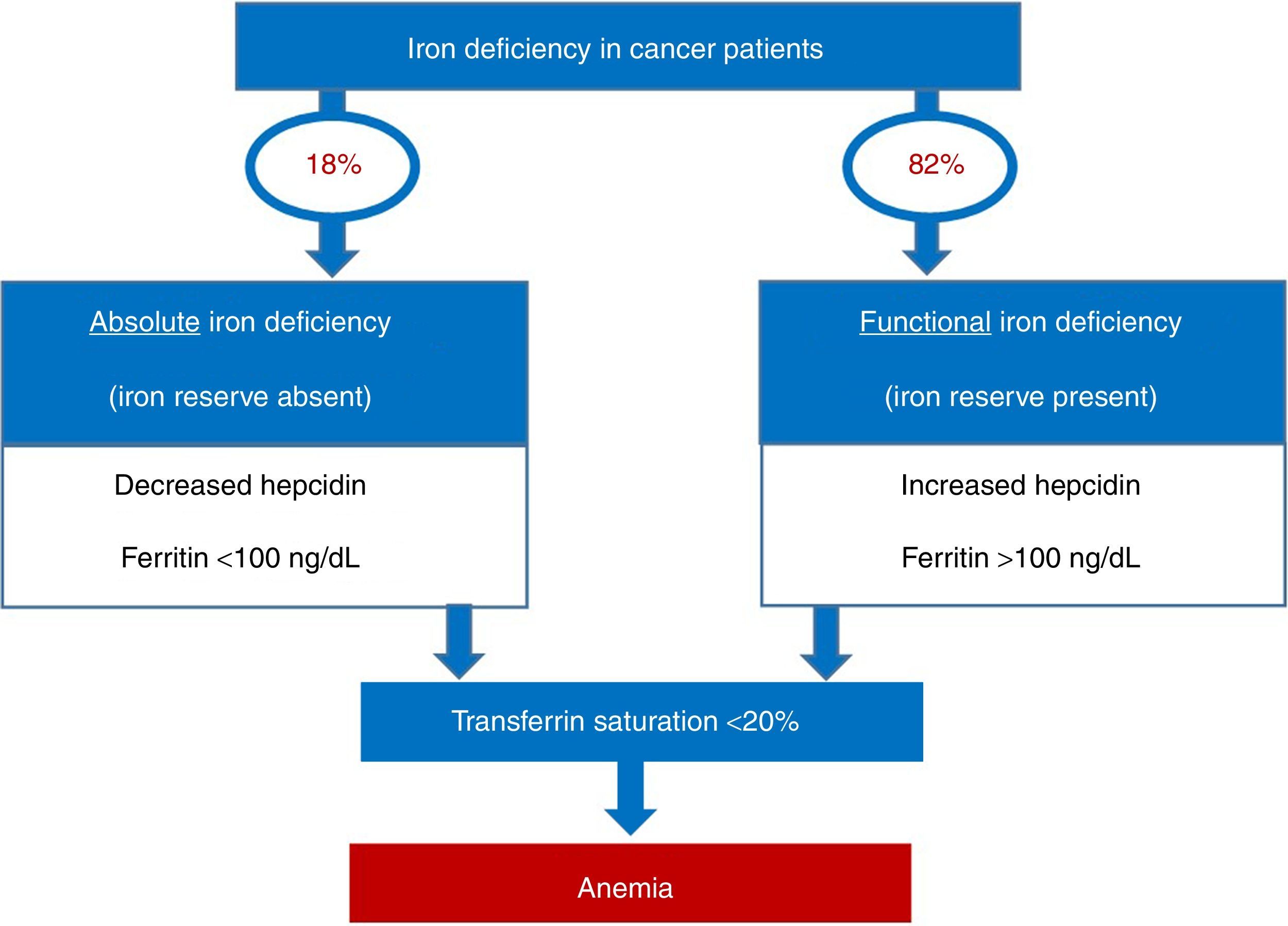
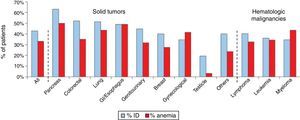
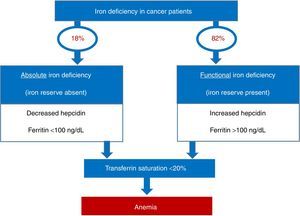
Iron deficiency can be classified as absolute, when iron reserves are depleted, or functional, when iron reserves are normal or even increased. The common event in both situations is the reduction of iron availability for erythropoiesis, leading to anemia. In absolute deficiency, for obvious reasons, the lack of iron in reserves is the main triggering event of anemia. In the case of functional iron deficiency (FID), although the reserves are satisfactory, the presence of an inflammatory process causes the iron to become ‘trapped’ in macrophages and enterocytes, limiting its availability to the bone marrow, triggering anemia. As expected, FID is the predominant mechanism of iron deficiency associated with cancer. The prevalence of FID in oncology patients ranges from 29 to 46%, and of iron deficiency associated to anemia ranges from 7 to 42%. Figure 1 shows the prevalence of iron deficiency and anemia in various types of solid tumors and hematologic malignancies.3
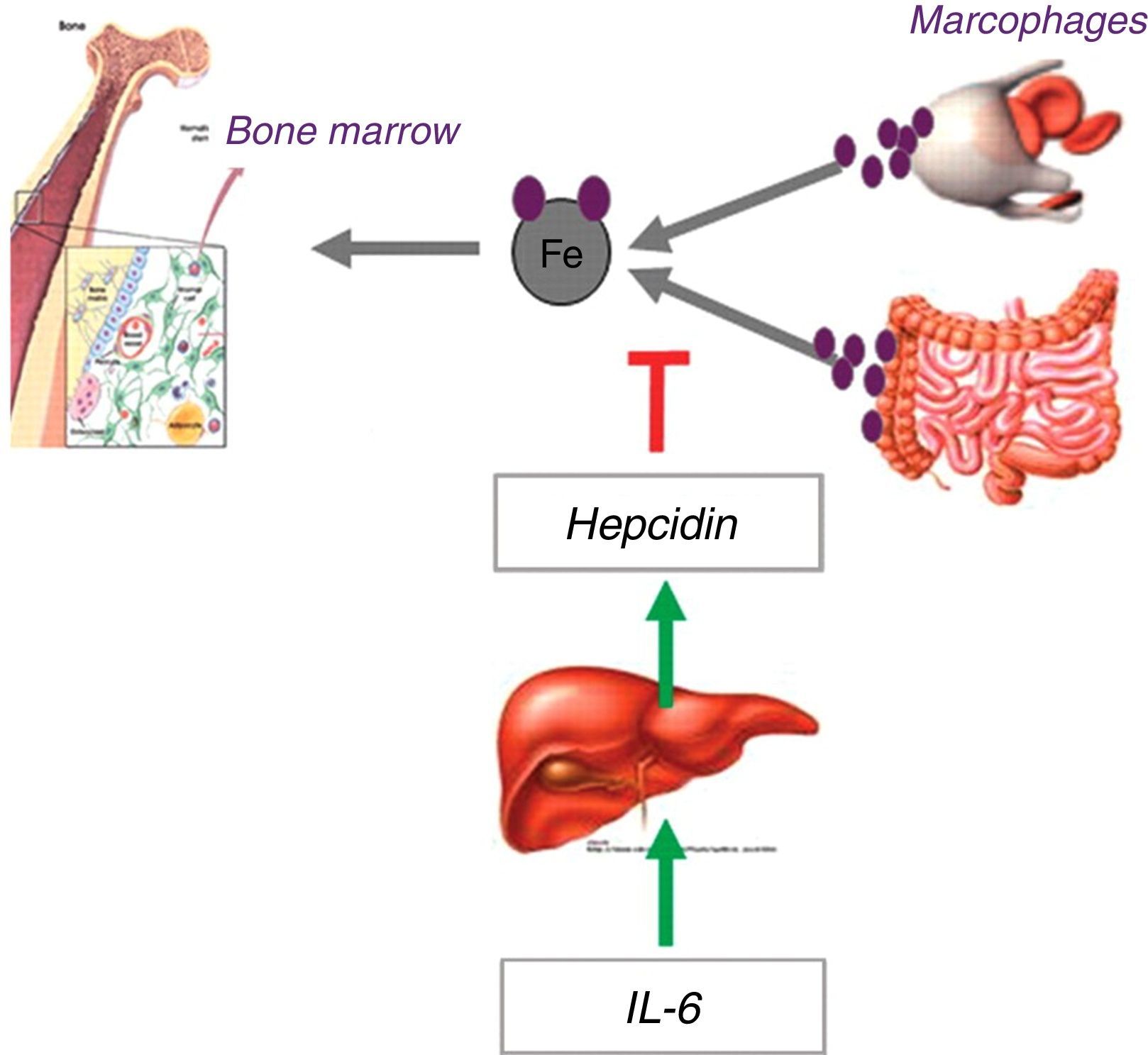
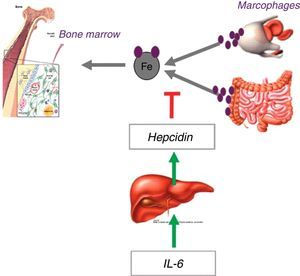
Functional iron deficiency is the predominant mechanism in cancer patientsThe demand for iron by the bone marrow and other tissues is supplied by the transport of this element in the circulation by transferrin. The iron taken up by transferrin can come from absorption by duodenal enterocytes or from recycling senescent erythrocytes by macrophages. Both intestinal absorption and macrophage release of iron are controlled by hepcidin, a regulatory hormone synthesized by the liver, which, under increased concentration, degrades the iron export protein, ferroportin, precluding the availability of iron for transportation from these locations.4
In the presence of an inflammatory process, iron availability is reduced to about 44% of the normal.5 This is due to the release of inflammatory cytokines, particularly interleukin-6, which activates the hepatic hepcidin transcription promoting genes, increasing hepcidin concentrations, and promoting the blockage of iron input into the circulation and reducing its availability (Figure 2).6
Thus, even in the presence of adequate iron reserves, cancer patients may develop lack of bioavailable iron, especially when treated with erythropoiesis stimulating agents (ESAs) that rapidly increase the production of red blood cells and the use of iron. This is the basic mechanism of functional iron deficiency, the prevalence of which increases in the more advanced stages of cancer and is associated with poor performance status (The Eastern Cooperative Oncology Group – ECOG).3
The importance of correct diagnosis… what is there besides ferritin?Traditionally, serum ferritin is the most widely used test for the diagnosis of iron deficiency, due to mirroring iron reserves. However, because it is a protein of the acute phase of inflammation, its diagnostic applicability is considerably impaired in the presence of acute or chronic inflammatory processes. In this context, transferrin saturation (TS) becomes more reliable for diagnostic purposes.7
It is remarkable that, in the clinical practice, only half of cancer patients with anemia are investigated as to iron profile and when they are, the investigation basically consists of ferritin measurements, whereas transferrin saturation is rarely used.8 This is a valid question, as among cancer patients with reduced transferrin saturation, more than 80% have normal or elevated ferritin values.3
Also useful, although less available for this purpose, are the measurements of reticulocyte hemoglobin content, determination of the percentage of hypochromic red blood cells in the sample, soluble transferrin receptor, among others.9
Figure 3 provides a practical algorithm for differentiation between absolute and functional iron deficiency.
Clinical impact and prognosis of iron deficiency in cancer patientsThe immediate complications of iron deficiency is the onset or worsening of pre-existing anemia, with varying clinical repercussions, including worsening of the quality of life of the patients. This usually affects the patients’ performance status, with the risk of jeopardizing their adherence to treatment, and may indirectly affect therapeutic results.3,10
The treatment of iron deficiency in cancer patients is now recommended by several guidelinesCurrently, the treatment of anemia in cancer patients basically consists of red blood cell transfusions, use of ESAs and iron supplementation. The main therapeutic goal is to improve the quality of life, in addition to trying to reduce the number of blood transfusions, which are associated with the risk of transmitting infections, hemolytic and non-hemolytic transfusion reactions, alloimmunization, etc.7,11
In this context, the use of ESAs and iron supplementation are effective treatment options. However, the response to ESAs in patients undergoing chemotherapy and radiation therapy ranges from 35 to 70%, and is limited by factors such as iron deficiency, concomitant infections, neoplastic activity and poor bone marrow reserve.7,11 Furthermore, some studies and meta-analyzes have raised the question of the risk of thromboembolic events with possible increases in mortality associated to the use of ESAs, especially with off-label indications in patients who are not on chemotherapy.12
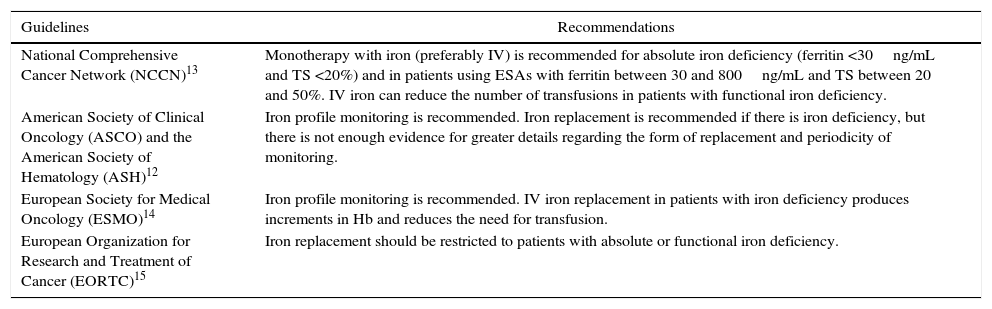
For these reasons, supplementation with iron stands as an attractive therapeutic option, included in several guidelines on the treatment of cancer patients with iron deficiency, either as monotherapy in order to improve anemia or in association with ESAs to enhance response to these agents (Table 1).
Recommendations on monitoring and iron replacement in cancer patients.
| Guidelines | Recommendations |
|---|---|
| National Comprehensive Cancer Network (NCCN)13 | Monotherapy with iron (preferably IV) is recommended for absolute iron deficiency (ferritin <30ng/mL and TS <20%) and in patients using ESAs with ferritin between 30 and 800ng/mL and TS between 20 and 50%. IV iron can reduce the number of transfusions in patients with functional iron deficiency. |
| American Society of Clinical Oncology (ASCO) and the American Society of Hematology (ASH)12 | Iron profile monitoring is recommended. Iron replacement is recommended if there is iron deficiency, but there is not enough evidence for greater details regarding the form of replacement and periodicity of monitoring. |
| European Society for Medical Oncology (ESMO)14 | Iron profile monitoring is recommended. IV iron replacement in patients with iron deficiency produces increments in Hb and reduces the need for transfusion. |
| European Organization for Research and Treatment of Cancer (EORTC)15 | Iron replacement should be restricted to patients with absolute or functional iron deficiency. |
IV: intravenous; TS: transferrin saturation; ESAs: erythropoiesis stimulating agents; Hb: hemoglobin.
There are several iron dosages and formulations available for treatment. In cancer patients, oral iron replacement is ineffective because intestinal iron absorption is considerably reduced in these patients, with more than 95% of this element being excreted in the feces. Furthermore, the small amount of absorbed iron is ‘locked’ within the enterocytes due to metabolic disorders induced by the previously mentioned inflammatory cytokines. Another factor that impairs the outcome is the decreased adherence to oral treatment because of the gastrointestinal side effects.7,16
Intravenous iron, on the other hand, can overcome the absorptive inflammatory blockade of iron, since the injected iron is directly captured by macrophages. In case of less stable complexes of intravenous iron, such as saccharate iron and ferric gluconate, the carbohydrate capsule that coats the iron is easily degraded and the iron is quickly released, requiring multiple low-dose infusions. However, with more stable complexes, such as ferric carboxymaltose (FCM), iron release is slow and prolonged, enabling the administration of high doses of iron in a single infusion. Thus, 1000mg of iron can be administered in a single 15-min infusion without the risk of releasing excessive iron, particularly as its free or toxic forms.7,11,16,17
Thus, FCM is one of the most widely used compounds in the treatment of iron deficiency in cancer patients, both in association with ESAs and as monotherapy.
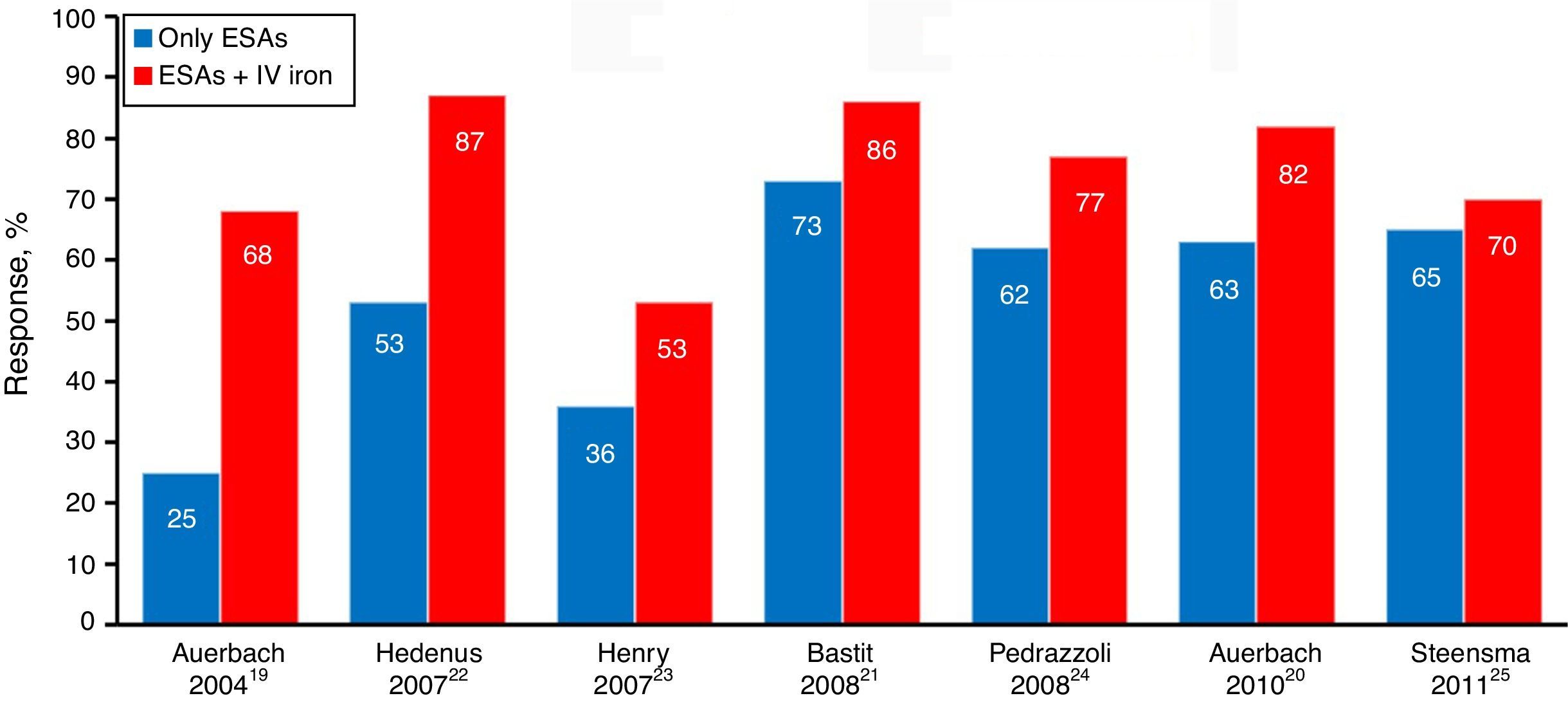
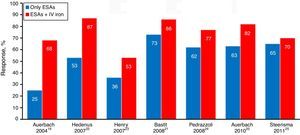
The main studies published so far, including a meta-analysis, favor the results of the association of ESAs and intravenous iron rather than ESA alone, because of the sharp increase in hematologic response evidenced by the increment in hemoglobin (Hb – Figure 4), faster response time, better quality of life, reduced need for transfusion and dose of ESAs when used in combination therapy.18–25
In addition to clinical benefits, the treatment of chemotherapy induced anemia using FCM was shown to be more cost effective, notably by reducing the use and dose of ESAs.26
Regarding the use of intravenous iron as monotherapy, an observational study of 639 solid tumor and onco-hematological patients showed similar increments in Hb levels with the use of FCM alone and FCM in association with ESAs (1.4g/dL and 1.6g/dL, respectively).27 Similarly, a single dose of 1000mg FCM in anemic patients (Hb 8.5–10.5g/dL, TS<20%, ferritin>40ng/mL in men and >30ng/mL in women) with low grade non-Hodgkin's lymphoma and prior chemotherapy compared to no treatment showed greater increase in Hb (2.5 vs. 0.9g/dL).28
A cost minimization analysis of patients scheduled for surgery for colorectal cancer, demonstrated that the use of FCM before surgery reduces direct and indirect costs of hospitalization compared to the use of saccharate iron and oral iron.29 This cost savings can be explained by the reduced need for transfusion and hospitalization time; the preoperative use of FCM was shown in another study of anemic patients in a similar scenario.30
ConclusionFunctional iron deficiency, triggered by the inflammatory process associated with cancer or its treatment, as well as by the use of ESAs, is considerably common in cancer patients. As a result, the iron profile should be investigated in anemia of cancer patients, in which decreased transferrin saturation, even with normal or high levels of ferritin, denotes functional iron deficiency.
The treatment of iron deficiency in oncology is already recommended by several specialty guidelines and should be carried out preferably with intravenous iron. The evidences regarding the efficacy of this treatment are solid, with a better response when combined with ESA and significant increments in Hb as monotherapy. Among the available intravenous iron formulations, FCM presents more favorable pharmacological characteristics with good efficacy in this inflammatory scenario, in addition to a more convenient dosing regimen and good cost-effective ratio, as a smaller number of transfusions and lower doses of ESA are required.
Conflicts of interestThe author declares no conflicts of interest.
I gratefully acknowledge the financial support of Takeda for the translation of this manuscript.