Neurolymphomatosis (NL) is a rare disease that can be caused by T cell, B cell or natural killer cell (NK) lymphomas, but most commonly by B cell lymphomas.1 NL is characterized by direct infiltration of the central nervous system (CNS), nerve roots/plexus or peripheral nerves by a hematological malignancy.2–4 Multiple nerve involvement is more common than single nerve involvement.1 Secondary NL occurs as relapse or the progression of lymphomas.5
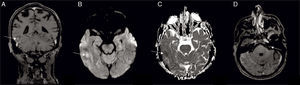
Case reportA 67-year-old male with a history of hepatic transplant in 2002 and under treatment with immunosuppressive agents (entecavir and everolimus), received the diagnosis of a diffuse large B-cell lymphoma (DLBCL) in May 2014 (lymph node conglomerate in the right armpit). The patient was treated with five cycles of rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP). In September, at the end of the treatment, the patient was admitted to an urgent care facility with left frontotemporal headaches related to eye and facial pain (V1 and V2 territory), as well as diplopia. Magnetic resonance imaging (MRI) showed parenchymal and pachymeningeal involvement in right temporal region, leptomeningeal thickening and enhancement of the oculomotor, trigeminal, facial, and vestibulocochlear cranial nerves (Figure 1). Cerebrospinal fluid (CSF) cytology was negative. The patient and the family refused the option of CNS biopsy, but opted for palliative radiotherapy in order to control the headache. The patient died in November 2014.
(A) Coronal T1-weighted magnetic resonance imaging after gadolinium administration showing right temporal leptomeningeal, parenchymal (arrow) and pachymeningeal enhancement; (B) diffusion weighted image with hyperintensity and (C) apparent diffusion coefficient map with hypointensity (arrow), confirming restricted diffusion; (D) axial T1-weighted magnetic resonance imaging after gadolinium administration, showing abnormal enhancement in the left facial nerve (arrow).
Primary NL is defined by neurological involvement as the initial manifestation of a hematological malignancy. Secondary NL occurs as relapse or progression of previously diagnosed lymphoma or leukemia.5 Symptoms of NL include sensorimotor deficits, muscular atrophy, hypotonia, hyporeflexia, spontaneous pain, headaches and cranial nerve dysfunction.2–4 The differential diagnoses include nerve damage from herpes zoster, chemotherapy, inflammatory neuropathy, drug-induced neuropathy, nerve root compression, radiotherapy, lymphoma-associated vasculitis, and paraneoplastic syndromes.1,6 A high index of suspicion is required due to the variety of symptoms and a large number of differential diagnoses that must be considered. NL is often misdiagnosed or undiagnosed due to its rarity and complex clinical manifestations.1,5
Diffuse infiltration of cranial nerves is the least common metastatic clinical presentation of NL.2–4 Ten percent of all metastatic nervous system lymphomas manifest as NL.3 In this article, we describe a rare case of secondary DLBCL with leptomeningeal and multiple cranial nerve involvement in an immunocompromised patient.
Magnetic resonance imaging (MRI) of NL includes abnormal enhancement of the leptomeninges, cranial nerves, or the periventricular region.7 The radiographic MRI appearance of NL includes contrast enhancement of peripheral nerves often with enlargement and nodularity. Involved nerves are isointense on T1-weighted MRI and hyperintense due to increased signal on short inversion time inversion recovery (STIR) and T2-weighted MRI.3 Leptomeningeal, subependymal, dural, or cranial nerve enhancement are findings suggestive of leptomeningeal metastases in neuroimaging tests.8 MRI contrast is the imaging technique of choice to detect leptomeningeal metastasis. Computerized tomography (CT) is less sensitive.8 Parenchymal metastases originating from non-Hodgkin lymphoma often appear as single or multiple enhanced lesions and may be accompanied by leptomeningeal metastases.8
Tumors are frequently more cellular than the tissue from which they originate therefore, they exhibit relatively high signal intensity and restricted diffusion is common for CNS lymphoma lesions.4,5 In MRI diffusion-weighted images, CNS lymphomas usually present a low apparent diffusion coefficient (ADC), featuring diffusion restriction.9 Involvement of cranial nerves can be seen, but this is relatively infrequent.9 When cranial nerves are affected, MRI typically shows enhancement of affected nerves, so it is the most used method to corroborate the diagnosis of NL.4
In cases with an appropriate clinical context, MRI with gadolinium is, by itself, adequate to establish the diagnosis of leptomeningeal metastasis.3 Nerve biopsy remains the gold standard in the diagnosis of NL, but it is not usually performed if noninvasive techniques are sufficient for diagnosis2. Positron emission tomography-computed tomography (PET-CT) is better than MRI and can often elucidate the diagnosis of NL when other diagnostic modalities are indeterminate.1,6 Cerebrospinal fluid examinations are not highly sensitive to diagnose NL. In a series of 96 patients with CNS lymphoma, 12 had MRI findings suggestive of leptomeningeal involvement, but only seven of these patients (58.3%) had positive CSF cytology.4 The diagnosis of secondary neurolymphomatosis was reached based on clinical data and MRI findings.
The prognosis of NL varies greatly among patients. The overall prognosis of patients with DLBCL has improved significantly with the addition of rituximab to the CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) regimen.10 Patients with lymphoma and NL have an overall median survival of 21 months and 15 months after the onset of NL symptoms.2 Treatment of NL is unsatisfactory and patients have poor outcome. The patient died two months after the beginning of NL symptoms. Currently, intravenous methotrexate is the first line therapy to treat NL due to its penetration of the blood–brain barrier. Additional treatments may be combined depending on the distribution and extent of disease.2
NL is an increasingly recognized complication of non-Hodgkin lymphoma and leukemia in patients undergoing therapy and its incidence is increasing worldwide, mainly due to increased awareness of this condition and early detection by more sophisticated diagnostic techniques.2 Early clinical recognition and treatment of NL, permits palliation and potentially retards neurological disease progression.3