Hematology Specialist Association 18. National Congress
Mais dadosAcute myeloid leukemia (AML) is a heterogeneous disease due to genetic abnormalities and differences in immunophenotypes. The diagnosis of AML requires a careful evaluation of clinical morphology, immunophenotyping, cytogenetics, and molecular analyses. 1 In current practice, flow cytometry-based immunophenotyping provides a rapid and reliable method for diagnosing AML, including acute promyelocytic leukemia (APL). APL is a subtype of AML with distinct morphological, biological, and clinical characteristics. It can be effectively treated with ATRA-based therapy protocols. However, if not treated quickly, it can be fatal due to the risk of disseminated intravascular coagulation (DIC). Therefore, the diagnosis and treatment of APL represent a true medical emergency.2
The absence of CD34, HLA-DR, and CD11b is a characteristic immunophenotypic feature that often distinguishes APL from other AML subtypes. However, AML subtypes other than APL that lack CD34 and HLA-DR expression have also been reported. APL accounts for 8% to 17% of AML patients. In AML patients without the PML-RARA fusion gene, 12% to 21% of cases have been identified as HLA-DR negative. These HLA-DR negative AML cases are distinct from APL because they do not carry the characteristic PML-RARA fusion.3
HLA-DR and CD34 negativity is generally observed in AML-M1 and AML-M2 subtypes and is associated with nucleophosmin (NPM1) gene mutations and FMS-like tyrosine kinase-internal tandem duplication (FLT3-ITD) mutations.4 NPM1 mutations are among the most common genetic abnormalities in AML, occurring in 27-35% of adult AML cases. Although rare, an "APL-like" immunophenotype has been reported in some de novo acute myeloid leukemia (AML) cases with NPM1 gene mutations. These cases show some immunophenotypic similarities to APL, despite being genetically different. AML cases with NPM1 mutations have unique clinical and biological characteristics.5
In this study, we aimed to highlight the association of FLT3-ITD positivity, as opposed to NPM1, in HLA-DR negative non-APL AML cases in our clinic.
MethodologyWe examined three acute leukemia patients who were referred to our clinic within one month and were initially reported as APL based on flow cytometry analysis. Our focus on these patients stemmed from the fact that a condition with an incidence of 1-2 cases per 1 million people per year was diagnosed consecutively as APL in flow cytometry analysis within a short period. Fluorescent in situ hybridization (FISH) analysis for the 15;17 translocation and polymerase chain reaction (PCR) for FLT3-ITD and FLT3-TKD mutations were performed on the patients' peripheral blood. NPM1 mutations could not be analyzed in these patients.
ResultsMorphological examination of the patients' peripheral blood smears showed prominent nucleoli, Auer rods, and cup-like nuclei. Due to the CD34 and HLA-DR negativity in the flow cytometry analysis, these cases were initially considered APL. However, cytogenetic results revealed a negative t(15;17) translocation in all three patients, excluding APL. Additionally, all three patients tested positive for FLT3-ITD mutations. The peripheral blood white blood cell (WBC) count, blast percentage, and D-dimer levels were significantly elevated at the time of presentation in all patients. (Table 1)
ConclusionIn cases with APL-like immunophenotypes, these similarities pose diagnostic challenges in daily practice. In this study, the APL-like AML cases exhibited CD34 and HLA-DR negativity and carried FLT3-ITD mutations. These de novo cases were characterized by high WBC counts, blast percentages, and elevated D-dimer levels.
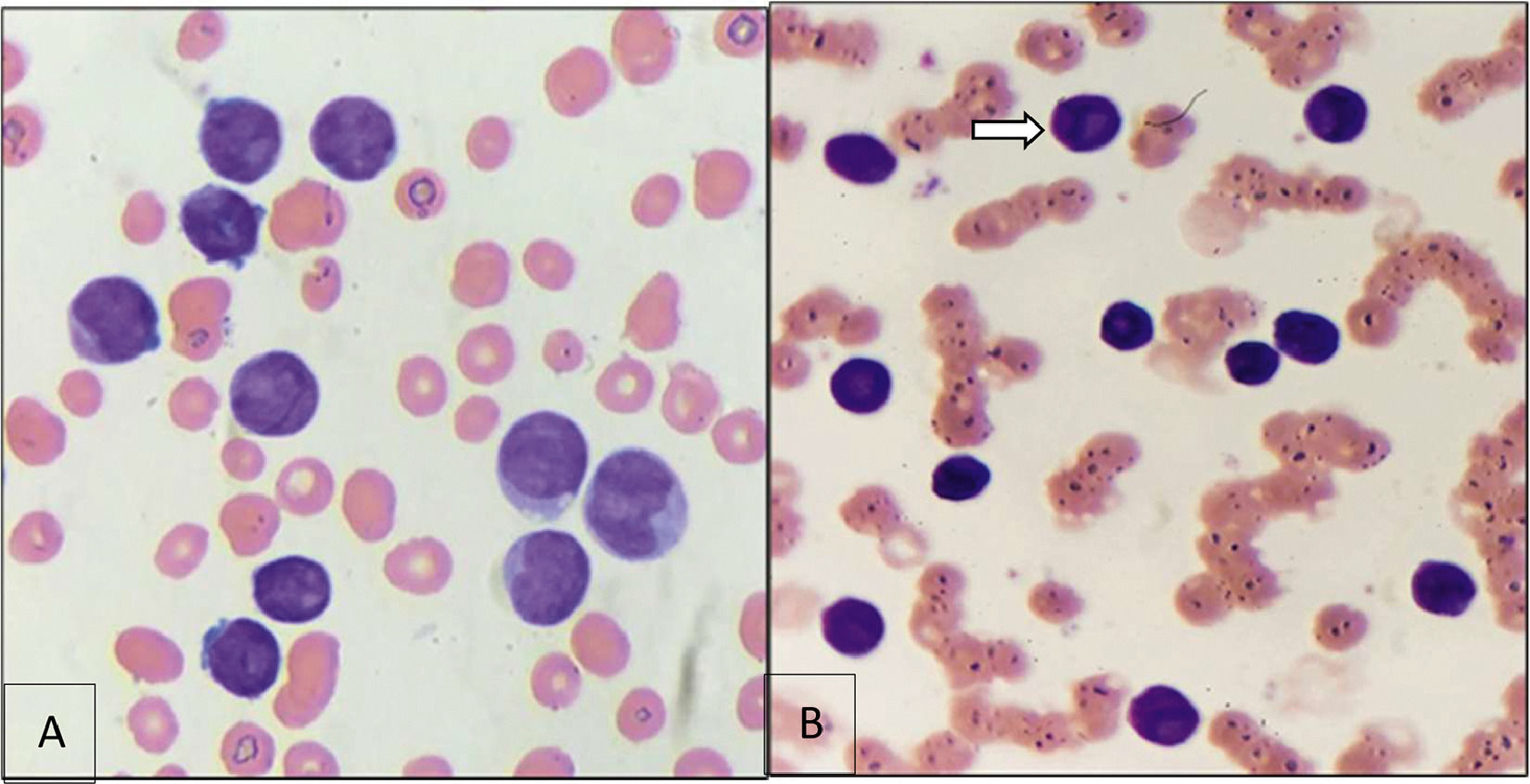
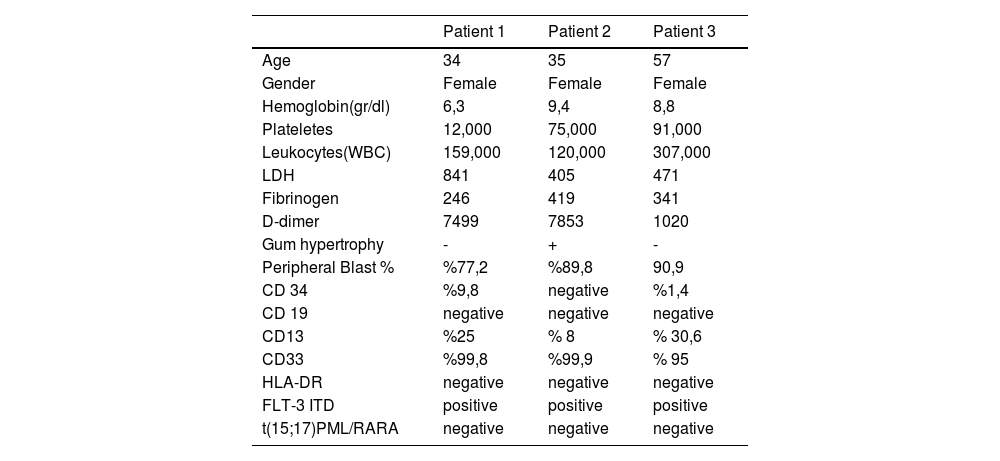
NPM1 is one of the most frequently mutated genes in AML, often seen alongside FLT3-ITD. Morphological and immunophenotypic similarities between many AML cases with NPM1 mutations and APL are well-known. In the first case, the blasts resembled the abnormal promyelocytes of APL (Figure 1.A). In the second case, a blast with a cup-like nucleus was observed (Figure 1.B). The "cup-like" nucleus morphology is specifically associated with acute myeloid leukemia (AML) with NPM1 gene mutations.
The WBC count was very high in all cases a feature that is unusual for APL, especially the hypergranular variant. High WBC counts and blast cell percentages are typically described in NPM1-mutated AML.6 Similarly, this could also be considered for FLT3-ITD, based on our findings. However, further studies with more cases are needed to confirm this. All patients demonstrated elevated D-dimer levels, which is more strongly associated with APL.7 Unlike high D-dimer levels, fibrinogen levels were within acceptable limits. One patient had prominent gum hypertrophy, and frequent gum bleeding was observed during clinical follow-up.
In conclusion, for cases with APL-like features but negative PML-RARA results by FISH and/or molecular methods, it is important to consider AML with NPM1 and/or FLT3-ITD mutations.
Clinical-Pathological Parameters of the Patients
Figure 1: A) Blast Cells B) "Cup-like" Nucleus Morphology