We evaluated possible relationships between echocardiographic findings and clinical and laboratory parameters, in a cohort of Brazilian patients diagnosed with sickle cell/β-thalassemia, to better understand the cardiac involvement in this disease.
ResultsLeft atrial (LA) and left ventricular (LV) dilation were found in 19.5 and 11% of patients, respectively; systolic left ventricular dysfunction was present in a single patient. There were no differences in masses and volumes of cardiac chambers comparing Sβ0 with Sβ+ patients, and no relationship between these parameters and specific complications of the disease. However, parameters of altered ventricular geometry were significantly correlated with serum creatinine, hepatic transaminases and bilirubin levels. Moreover, 3 patients presented stroke; they were significantly older [53 (41–56)×37.5 (18–70), p=0.048], had higher values of LV posterior wall diastolic thickness [10 (10–11)×8 (6–14), p=0.03], LV mass [226 (194–260)×147 (69–537), p=0.039] and LA/aortic ratio [1.545 (1.48–1.61)×1.26 (0.9–1.48), p=0.032].
ConclusionsCardiac involvement in this disease does not appear to depend on the thalassemia phenotype. The presence of signs of myocardial remodeling in this group of patients was related to multi-organ impairment and rendered a higher propensity for stroke in older patients, suggesting the need for greater vigilance and control of associated factors.
The double heterozygosity of sickle cell disease (SCD) and beta-thalassemia (sickle cell/β-thalassemia or HbS/β-thal) leads to the clinical expression of a variant form of SCD, with multiple acute and chronic complications, such as painful vaso occlusive events, cerebral vasculopathy, priapism, renal and lung disease, among others.1 Disease severity varies among patients, even between individuals with a similar genotype, but depends highly on the degree of the beta-thalassemia mutation: decreased (β+) or absent (β0) beta-globin synthesis.2
This condition has a considerable prevalence in Brazil, due to the intense miscegenation of this population, including Mediterranean Caucasians and Afro-descendants, among other ethnic groups, creating a very peculiar genetic profile. However, although much is known regarding the organic involvement in SCD and beta-thalassemia, there are few studies specifically evaluating sickle cell/β-thalassemia patients.
Our group has previously identified significant differences among sickle cell/β-thalassemia patients according to the beta globin mutation.3 The Sβ+ individuals are more prone to acute chest syndrome, and Sβ0 patients presented with a lower body mass index (BMI) and bone mineral density. The degree of bone damage correlates to lower BMI and hemoglobin levels, as well as plaquetosis, monocytosis and elevated lactate dehydrogenase (LDH), possibly reflecting the effects of hemolysis and inflammation on bone metabolism and body constitution.3
Heart disease represents an important cause of mortality and morbidity in sickle cell disease and beta-thalassemia patients.4 However, the knowledge on the involvement of cardiac function in this specific group of compound heterozygotes is scarce. Two studies evaluated the register of repercussions on this organ, both performed in Greece. Moyssakis et al. evaluated 43 patients with sickle β-thalassemia and 55 healthy controls and demonstrated that in the anemic group, the diastolic function was abnormal in both ventricles, whereas the systolic function remained unchanged.5 Aessopos et al. also showed biventricular dilatation and dysfunction in sickle beta-thalassemia patients, along with pulmonary hypertension, leading to congestive heart failure.6
Therefore, the objective of this study was to evaluate the relationship of echocardiographic findings with clinical and laboratory parameters in a cohort of Brazilian patients diagnosed with sickle cell/β-thalassemia, to better understand the cardiac involvement in this disease in our particular ethnic background.
Patients and methodsPatients and data collectionA retrospective chart review was made for patients with a diagnosis of sickle cell/β-thalassemia, regularly followed at the outpatient clinic of the Hematology and Transfusion Medicine Center of the University of Campinas, Brazil, from 1998 to 2016, comprising a total of forty-one patients (27 Sβ0 and 14 Sβ+). Patients were evaluated at steady-state disease and were transfused sporadically. All patients were informed regarding the purpose and procedures of the study and provided written consent. The medical records of these 41 patients were reviewed for laboratory and clinical data comprising age, gender, weight, height, body mass index (BMI=weight (kg)/height2 (m2)), white blood cell (WBC) counts, hemoglobin levels, reticulocyte counts, mean cell volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet counts, lactate dehydrogenase (LDH) level, hepatic enzymes and aspartate aminotransferase (AST); alanine aminotransferase (ALT); alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT), serum ferritin, serum iron, total iron-binding capacity (TIBC), haptoglobin, conjugated and unconjugated bilirubin levels, microalbuminuria, creatinine and glomerular filtration rate estimated by 51Cr-EDTA clearance. Hospital admissions were recorded for all sickle related organ involvements, especially acute chest syndrome (ACS), which was recorded according to the current criteria: new infiltrate visible on chest radiograph associated with one or more symptoms, such as fever, cough, tachypnea, breathing difficulties or new hypoxia onset; retinopathy as the presence of a dense capillary bed up to the margin of perfusion, with abrupt termination of small or medium caliber vessels and irregular appearance of the border; avascular bone necrosis defined by a history of osteonecrosis of the femoral head or joint replacement; stroke defined by patient reported cerebrovascular disease (when treated in other emergency services) or documented by CT or MRI at our center. Episodes of priapism, leg ulcers and venous thromboembolism (VTE) were also carefully recorded. Laboratory tests were performed as part of a routine evaluation during the steady-state condition.
Echocardiographic evaluationEchocardiographic evaluation was performed on all patients at rest, in steady-state disease, with pulsed, continuous, and color Doppler, with Hewlet Packard Sonos 1000 ultrasound System, using a 2.5MHz transducer. All studies were done by two observers unaware of the subject's status. A complete M-mode, two-dimensional examination was used to assess: LV systolic and diastolic wall thickness (SWTh and DWTh, respectively), LV end diastolic and systolic diameters (EDD and ESD) and end systolic volumes (ESV). The following indexes were calculated: LV percent fractional shortening (%FS), and LV percent ejection fraction (%EF). All parameters were normalized per m2 body surface area (BSA). A continuous wave Doppler echocardiogram was used to estimate the peak systolic pressure gradient across the tricuspid valve as an indicator of pulmonary hypertension.
Statistical analysisExploratory data analyses were used to investigate the distribution of variables. The means and standard deviations (SD) or median and range were used to summarize data, when applicable. The relationships among variables were tested for significance using Spearman's rank correlation and Wilcoxon/Kruskal–Wallis Rank Sum tests. Fisher's Exact Test was used for categorical variables. The Wilcoxon/Kruskal–Wallis Rank Sum and Fisher's Exact tests were used for comparisons between the two groups (Sβ0 and Sβ+) and Spearman's rank correlation test were used to evaluate relationships among variables in the whole group of patients. Results were considered significant at p<0.05. Statistical analysis was performed using the R software version 3.3.0 (2016-05-03) Copyright (C) 2016, The R Foundation for Statistical Computing.
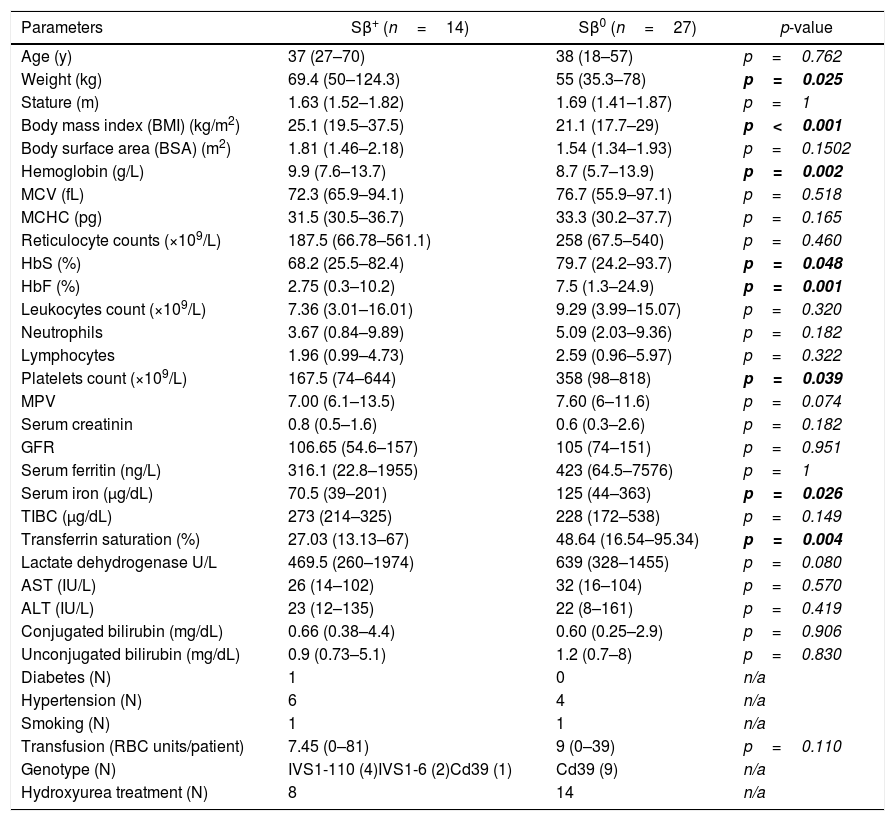
ResultsDemographic findings and clinical featuresAmong the studied population, 23 (56%) were women and 18 (44%) were men. The median age was 38 years, with a range of 18–70 years; 27 (65%) patients were diagnosed as Sβ0, and 14 (35%) as Sβ+. The patients’ clinical and laboratory characteristics described in this study are detailed in Table 1.
Patients’ clinical and laboratory data.
| Parameters | Sβ+ (n=14) | Sβ0 (n=27) | p-value |
|---|---|---|---|
| Age (y) | 37 (27–70) | 38 (18–57) | p=0.762 |
| Weight (kg) | 69.4 (50–124.3) | 55 (35.3–78) | p=0.025 |
| Stature (m) | 1.63 (1.52–1.82) | 1.69 (1.41–1.87) | p=1 |
| Body mass index (BMI) (kg/m2) | 25.1 (19.5–37.5) | 21.1 (17.7–29) | p<0.001 |
| Body surface area (BSA) (m2) | 1.81 (1.46–2.18) | 1.54 (1.34–1.93) | p=0.1502 |
| Hemoglobin (g/L) | 9.9 (7.6–13.7) | 8.7 (5.7–13.9) | p=0.002 |
| MCV (fL) | 72.3 (65.9–94.1) | 76.7 (55.9–97.1) | p=0.518 |
| MCHC (pg) | 31.5 (30.5–36.7) | 33.3 (30.2–37.7) | p=0.165 |
| Reticulocyte counts (×109/L) | 187.5 (66.78–561.1) | 258 (67.5–540) | p=0.460 |
| HbS (%) | 68.2 (25.5–82.4) | 79.7 (24.2–93.7) | p=0.048 |
| HbF (%) | 2.75 (0.3–10.2) | 7.5 (1.3–24.9) | p=0.001 |
| Leukocytes count (×109/L) | 7.36 (3.01–16.01) | 9.29 (3.99–15.07) | p=0.320 |
| Neutrophils | 3.67 (0.84–9.89) | 5.09 (2.03–9.36) | p=0.182 |
| Lymphocytes | 1.96 (0.99–4.73) | 2.59 (0.96–5.97) | p=0.322 |
| Platelets count (×109/L) | 167.5 (74–644) | 358 (98–818) | p=0.039 |
| MPV | 7.00 (6.1–13.5) | 7.60 (6–11.6) | p=0.074 |
| Serum creatinin | 0.8 (0.5–1.6) | 0.6 (0.3–2.6) | p=0.182 |
| GFR | 106.65 (54.6–157) | 105 (74–151) | p=0.951 |
| Serum ferritin (ng/L) | 316.1 (22.8–1955) | 423 (64.5–7576) | p=1 |
| Serum iron (μg/dL) | 70.5 (39–201) | 125 (44–363) | p=0.026 |
| TIBC (μg/dL) | 273 (214–325) | 228 (172–538) | p=0.149 |
| Transferrin saturation (%) | 27.03 (13.13–67) | 48.64 (16.54–95.34) | p=0.004 |
| Lactate dehydrogenase U/L | 469.5 (260–1974) | 639 (328–1455) | p=0.080 |
| AST (IU/L) | 26 (14–102) | 32 (16–104) | p=0.570 |
| ALT (IU/L) | 23 (12–135) | 22 (8–161) | p=0.419 |
| Conjugated bilirubin (mg/dL) | 0.66 (0.38–4.4) | 0.60 (0.25–2.9) | p=0.906 |
| Unconjugated bilirubin (mg/dL) | 0.9 (0.73–5.1) | 1.2 (0.7–8) | p=0.830 |
| Diabetes (N) | 1 | 0 | n/a |
| Hypertension (N) | 6 | 4 | n/a |
| Smoking (N) | 1 | 1 | n/a |
| Transfusion (RBC units/patient) | 7.45 (0–81) | 9 (0–39) | p=0.110 |
| Genotype (N) | IVS1-110 (4)IVS1-6 (2)Cd39 (1) | Cd39 (9) | n/a |
| Hydroxyurea treatment (N) | 8 | 14 | n/a |
Abbreviations: MCV: mean corpuscular volume, MCHC: mean corpuscular hemoglobin concentration, MPV: mean platelet volume, TIBC: total iron binding capacity, AST: aspartate aminotransferase, ALT: alanine aminotransferase, GFR: glomerular filtration rate (estimated by 51Cr-EDTA clearance), RBC: packed red blood cells.
Considering the Sβ-thalassemia phenotype, as expected, the two groups of patients differed significantly. Hemoglobin levels were significantly lower in Sβ0 patients, who also presented a higher proportion of HbS and HbF. In addition, these patients showed higher index levels of transferrin saturation and serum iron, probably reflecting a higher degree of ineffective hematopoiesis and/or hemolysis, despite the fact that other hemolysis parameters did not differ significantly between the two groups. Moreover, Sβ0 patients had a significantly lower body mass index and weight and a higher number of platelets, emphasizing the disease severity in this group of patients.
Of the Sβ+ patients, 42.8% had a previous diagnosis of Systemic Arterial Hypertension, as opposed to 14.8% of the Sβ0 patients. However, all of these patients were treated with antihypertensive medications, presenting satisfactory blood pressure control according to the means of blood pressure measurements in the last 3 visits: median systolic blood pressure of 113.5mmHg (88–129) in Sβ+ and 118 (102–128) in Sβ0 patients and median diastolic blood pressure: 66.5mmHg (52–80) in Sβ+ and 75 (59–91) in Sβ0 patients.
When we evaluated the median number of packed red blood cells (RBCs) transfused per patient in each group, we observed, as expected, a higher number of transfused RBCs per patient in the Sβ0 group (9 vs 7.45); however, this difference was not significant (p=0.11).
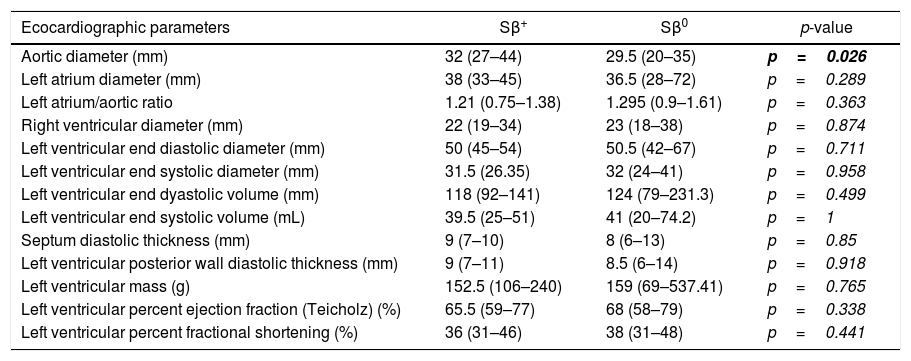
Evaluation of echocardiographic parameters in the cohort of patients with Sβ-thalassemiaExcept for the aorta diameter, which was higher in the Sβ0 patients [32 (27–44)×29.5 (20–35), p=0.026], there were no significant differences in cardiac parameters (ejection fraction, diameters, thicknesses, masses and volumes of cardiac chambers), comparing both groups of patients (Table 2).
Comparison of echocardiographic parameters according to the Sβ-thalassemia phenotype.
| Ecocardiographic parameters | Sβ+ | Sβ0 | p-value |
|---|---|---|---|
| Aortic diameter (mm) | 32 (27–44) | 29.5 (20–35) | p=0.026 |
| Left atrium diameter (mm) | 38 (33–45) | 36.5 (28–72) | p=0.289 |
| Left atrium/aortic ratio | 1.21 (0.75–1.38) | 1.295 (0.9–1.61) | p=0.363 |
| Right ventricular diameter (mm) | 22 (19–34) | 23 (18–38) | p=0.874 |
| Left ventricular end diastolic diameter (mm) | 50 (45–54) | 50.5 (42–67) | p=0.711 |
| Left ventricular end systolic diameter (mm) | 31.5 (26.35) | 32 (24–41) | p=0.958 |
| Left ventricular end dyastolic volume (mm) | 118 (92–141) | 124 (79–231.3) | p=0.499 |
| Left ventricular end systolic volume (mL) | 39.5 (25–51) | 41 (20–74.2) | p=1 |
| Septum diastolic thickness (mm) | 9 (7–10) | 8 (6–13) | p=0.85 |
| Left ventricular posterior wall diastolic thickness (mm) | 9 (7–11) | 8.5 (6–14) | p=0.918 |
| Left ventricular mass (g) | 152.5 (106–240) | 159 (69–537.41) | p=0.765 |
| Left ventricular percent ejection fraction (Teicholz) (%) | 65.5 (59–77) | 68 (58–79) | p=0.338 |
| Left ventricular percent fractional shortening (%) | 36 (31–46) | 38 (31–48) | p=0.441 |
The left atrial (LA) and left ventricular (LV) dilation were found in 19.5 and 11% of patients, respectively. Systolic LV dysfunction (defined as LV ejection fraction <55%) was present in a single patient of our cohort. Thus, we can observe a group of patients with LA enlargement and LV remodeling, but cardiac involvement in Sβ-thalassemia patients does not appear to be dependent on the thalassemia phenotype.
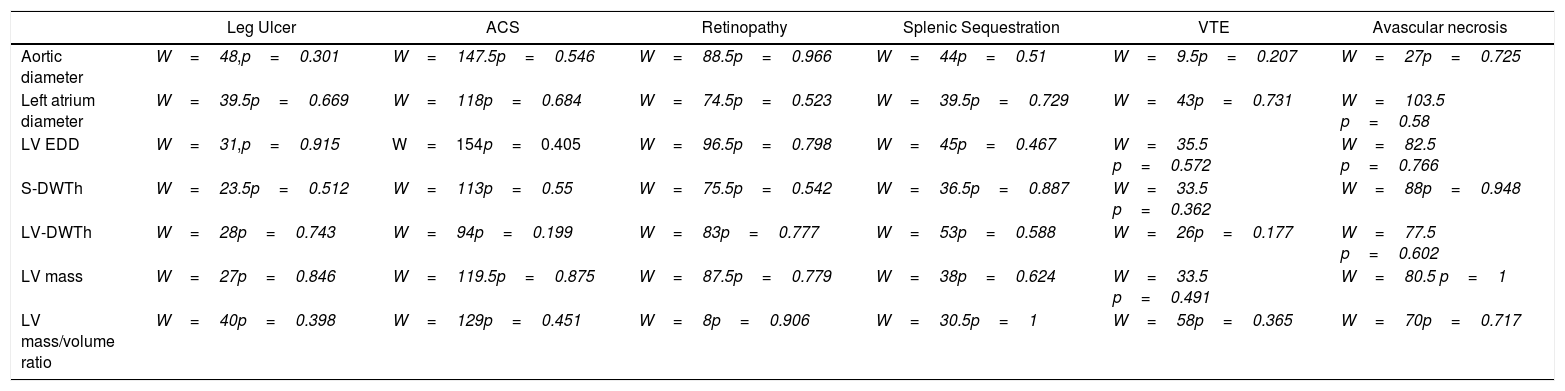
Association between ventricular geometry, dysfunction of target organs and strokeWe found no relationship between the echocardiographic parameters analyzed and the occurrence of specific complications of the disease (acute thoracic syndrome, leg ulcers, retinopathy, splenic sequestration, venous thromboembolism and avascular necrosis of the hip (Table 3).
Relationship between echocardiographic findings and specific complications of the disease (Wilcoxon rank sum test with continuity correction).
| Leg Ulcer | ACS | Retinopathy | Splenic Sequestration | VTE | Avascular necrosis | |
|---|---|---|---|---|---|---|
| Aortic diameter | W=48,p=0.301 | W=147.5p=0.546 | W=88.5p=0.966 | W=44p=0.51 | W=9.5p=0.207 | W=27p=0.725 |
| Left atrium diameter | W=39.5p=0.669 | W=118p=0.684 | W=74.5p=0.523 | W=39.5p=0.729 | W=43p=0.731 | W=103.5 p=0.58 |
| LV EDD | W=31,p=0.915 | W=154p=0.405 | W=96.5p=0.798 | W=45p=0.467 | W=35.5 p=0.572 | W=82.5 p=0.766 |
| S-DWTh | W=23.5p=0.512 | W=113p=0.55 | W=75.5p=0.542 | W=36.5p=0.887 | W=33.5 p=0.362 | W=88p=0.948 |
| LV-DWTh | W=28p=0.743 | W=94p=0.199 | W=83p=0.777 | W=53p=0.588 | W=26p=0.177 | W=77.5 p=0.602 |
| LV mass | W=27p=0.846 | W=119.5p=0.875 | W=87.5p=0.779 | W=38p=0.624 | W=33.5 p=0.491 | W=80.5 p=1 |
| LV mass/volume ratio | W=40p=0.398 | W=129p=0.451 | W=8p=0.906 | W=30.5p=1 | W=58p=0.365 | W=70p=0.717 |
Abbreviations: ACS: acute chest syndrome, VTE: venous thromboembolism, LV EDD: left ventricular end diastolic diameter, S-DWTh: septum diastolic thickness, LV-DWTh: LV diastolic wall thickness.
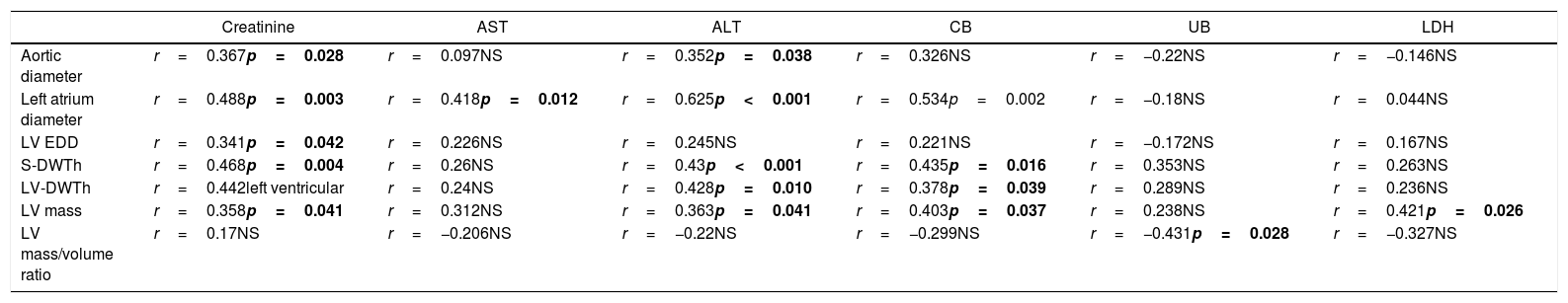
However, a significant correlation was found regarding parameters of ventricular geometry with serum creatinine, hepatic transaminases, bilirubin levels and LDH. This fact may indicate a progressive systemic dysfunction in this group of patients, involving a multi-organ involvement. These associations are shown in Table 4.
Relationship between echocardiographic findings and serum creatinine, AST, ALT and bilirubin levels (“r” values for Spearman correlation results, significant at p<0.05).
| Creatinine | AST | ALT | CB | UB | LDH | |
|---|---|---|---|---|---|---|
| Aortic diameter | r=0.367p=0.028 | r=0.097NS | r=0.352p=0.038 | r=0.326NS | r=−0.22NS | r=−0.146NS |
| Left atrium diameter | r=0.488p=0.003 | r=0.418p=0.012 | r=0.625p<0.001 | r=0.534p=0.002 | r=−0.18NS | r=0.044NS |
| LV EDD | r=0.341p=0.042 | r=0.226NS | r=0.245NS | r=0.221NS | r=−0.172NS | r=0.167NS |
| S-DWTh | r=0.468p=0.004 | r=0.26NS | r=0.43p<0.001 | r=0.435p=0.016 | r=0.353NS | r=0.263NS |
| LV-DWTh | r=0.442left ventricular | r=0.24NS | r=0.428p=0.010 | r=0.378p=0.039 | r=0.289NS | r=0.236NS |
| LV mass | r=0.358p=0.041 | r=0.312NS | r=0.363p=0.041 | r=0.403p=0.037 | r=0.238NS | r=0.421p=0.026 |
| LV mass/volume ratio | r=0.17NS | r=−0.206NS | r=−0.22NS | r=−0.299NS | r=−0.431p=0.028 | r=−0.327NS |
Abbreviations: AST: aspartate aminotransferase, ALT: alanine aminotransferase, CB: conjugated bilirubin, UB: unconjugated bilirubin, LV EDD: left ventricule end diastolic diameter, S-DWTh: septum diastolic thickness, LV-DWTh: LV diastolic wall thickness, NS: statistically not significant.
Among the 41 patients studied, 3 of them presented stroke (1 Sβ+ and 2 Sβ0). These patients were significantly older than the others (median 53 years×37.5 years, p=0.048), which may reflect a longer time of exposure to the organic damage caused by the disease. Patients affected by stroke had higher values of left ventricular posterior wall diastolic thickness [10 (10–11)×8 (6–14), p=0.03] and greater left ventricular mass [226 (194–260)×147 (69–537), p=0.039]. In addition, Sβ0 patients affected by stroke also presented a significantly higher left atrium/aortic ratio [1.545 (1.48–1.61)×1.26 (0.9–1.48), p=0.032].
Evaluation of pulmonary hypertensionNo significant differences were found in pulmonary artery systolic pressure values between Sβ+ and Sβ0 patients. In this small sample, we found no relationship between PASP and the occurrence of acute or chronic complications (acute chest syndrome, thromboembolism, stroke, retinopathy, splenic sequestration and avascular hip necrosis).
DiscussionImprovement in the care of patients with hemoglobinopathies during the last decades has increased their life expectancy, creating new challenges in the clinical management of these individuals. These include the possibility of deterioration of the cardiac function, which can add considerable morbidity to the clinical picture of the disease. Less common variants, such as sickle cell/beta-thalassemia, still lack better characterization in this topic, and the objective of this study was to evaluate the echocardiographic findings in this group of patients, seeking correlations with laboratory data and other complications.
Except for the aorta diameters (higher in the Sβ0 group), there were no significant differences in the other parameters (diameters, thicknesses, masses and volumes of cardiac chambers) between Sβ0 and Sβ+ patients. We therefore consider that the cardiac involvement in this disease does not depend so much on the thalassemia phenotype, unlike other clinical parameters, such as the body mass index and bone mineral density, which differ significantly between Sβ0 and Sβ+ patients.3 Cardiac involvement seems to occur more homogeneously in this group of individuals.
The prevalence of the left atrial and left ventricular dilation in our cohort was 19.5 and 11%, respectively. This prevalence is considerably lower than that reported for homozygous SS patients (78 and 35%, respectively),7 and is in agreement with the data found in Sβ-thalassemia patients of Greek origin.6 Although we do not have a more accurate evaluation of the occurrence of diastolic dysfunction in our group of patients, the dilation of chambers (mainly the LA) can be considered an indirect parameter of diastolic dysfunction.8 Previous studies have demonstrated a significant relation between the LA remodeling and echocardiographic indices of diastolic function9 and a higher LA volume index has shown to be an independent predictor of death, heart failure, atrial fibrillation, and ischemic stroke.10
New insights into the pathophysiology of heart disease in patients with sickle cell anemia demonstrate the association between myocardial fibrosis and the occurrence of diastolic dysfunction.11 Despite this mechanism not having been evaluated in sickle cell/β-thalassemia patients, this event may also be responsible for the alterations found in this group of compound heterozygotes. In fact, one limitation in our study was the unavailability of methods that can accurately evaluate the presence of still incipient cardiac lesions. An example would be the assessment of the global longitudinal strain (GLS), assessed by 2-dimensional speckle tracking echocardiography, a method able to recognize the cardiac involvement in early stages of disease.12 The GLS is altered despite preserved LV function, as assessed by the ejection fraction and seems to be helpful in the prediction of cardiovascular outcomes in the general population.13 These methods have already been evaluated specifically in patients with β-thalassemia, demonstrating an important correlation even with cardiac damage secondary to iron overload.14 They also present a better prognostic value for diastolic dysfunction in this group of patients than parameters evaluated in traditional echocardiography.15 The use of these new techniques in heterozygous patients still needs to be evaluated in future studies.
Systolic LV dysfunction (defined as LV ejection fraction <59%) was present in only one patient of our cohort, and it is also uncommon in SS patients (8.5% in the study of Damy et al.).7 Interestingly, a significant correlation was found regarding altered parameters of ventricular geometry with markers of systemic disease (serum creatinine, hepatic transaminases and bilirubin levels). Moreover, 3 of the 41 patients studied presented stroke (1 Sβ+ and 2 Sβ0), and these patients presented significantly higher values of left ventricular posterior wall diastolic thickness and greater left ventricular mass, and the 2 Sβ0 patients affected by stroke also presented a significantly higher left atrium/aortic ratio. These results may suggest a higher propensity for stroke in older patients with signs of ventricular hypertrophy.
Thus, in the case of Brazilian patients diagnosed with sickle cell/β-thalassemia, we can observe a higher prevalence of left atrial and left ventricular dilation with little systolic function impairment. In addition, the comparison between the two groups of patients reveals that cardiac involvement in this disease does not appear to depend on the thalassemia phenotype. The presence of signs of myocardial remodeling in this group of patients was related to multi-organ impairment, rendering a higher propensity for stroke in older patients, suggesting the need for greater vigilance and control of associated factors, such as hypertension, dyslipidemia and diabetes.
Conflicts of interestThe authors declare no conflicts of interest.