l-Asparaginase is essential in the treatment of childhood acute lymphoblastic leukemia. If immunoglobulin G anti-l-asparaginase antibodies develop, they can lead to faster plasma clearance and reduced efficiency as well as to hypersensitivity reactions, in which immunoglobulin E can also participate. This study investigated the presence of immunoglobulin G and immunoglobulin E anti-l-asparaginase antibodies and their clinical associations.
MethodsUnder 16-year-old patients at diagnosis of B-cell acute lymphoblastic leukemia confirmed by flow cytometry and treated with a uniform l-asparaginase and chemotherapy protocol were studied. Immunoglobulin G anti-l-asparaginase antibodies were measured using an enzyme-linked immunosorbent assay. Intradermal and prick skin testing was performed to establish the presence of specific immunoglobulin E anti-l-asparaginase antibodies in vivo. Statistical analysis was used to investigate associations of these antibodies with relevant clinical events and outcomes.
ResultsFifty-one children were studied with 42 (82.35%) having anti-l-asparaginase antibodies. In this group immunoglobulin G antibodies alone were documented in 10 (23.8%) compared to immunoglobulin E alone in 18 (42.8%) patients. Immunoglobulin G together with immunoglobulin E were simultaneously present in 14 patients. Children who produced exclusively immunoglobulin G or no antibodies had a lower event-free survival (p-value=0.024). Eighteen children (35.3%) relapsed with five of nine of this group who had negative skin tests suffering additional relapses (range: 2–4), compared to none of the nine children who relapsed who had positive skin tests (p-value<0.001).
ConclusionChildren with acute lymphoblastic leukemia and isolated immunoglobulin G anti-l-asparaginase antibodies had a higher relapse rate, whereas no additional relapses developed in children with immunoglobulin E anti-l-asparaginase antibodies after the first relapse.
Escherichia colil-asparaginase is key in the treatment of childhood acute lymphoblastic leukemia (ALL).1 High-intensity l-asparaginase regimens result in better outcomes than lower-dose schemes.2 The intravenous or intramuscular route can be used to administer l-asparaginase; the latter is well tolerated and does not appear to result in increased hypersensitivity reactions3 whereas the former is more immunogenic.4 More recently it was shown that the intravenous administration of pegylated l-asparaginase is also associated with a higher risk of allergic reactions.5 The l-asparaginase molecule is highly reactive, has a complex quaternary structure and elevated molecular weight and it can elicit production of immunoglobulin (Ig)G anti-l-asparaginase antibodies. These antibodies can cause severe allergic and hypersensitivity reactions, albeit rarely fatal, in children suffering a severe reaction, mostly mediated by IgG and complement.3,6 In these cases, substitution for l-asparaginase conjugated covalently with 5000 molecular weight polyethylene glycol is indicated, although one third of those switched to the pegylated enzyme still have allergic reactions due to the fact that the source of both preparations is the same bacterium.7,8 Interestingly, treatment with the enzyme derived from Erwinia chrysanthemi, which can substitute the typical variety of Escherichia coli, may not be necessary for some children with severe allergies to E. colil-asparaginase who have received at least half of intended doses.9 Important aspects for better therapeutic results and less frequent side effects include new sources of l-asparaginase to increase its availability, improved pharmacodynamics and pharmacokinetics and safer toxicological profile.10
Decreased efficacy of l-asparaginase due to high titers of IgG antibodies may be due to neutralizing antibodies, increased enzyme clearance, delayed absorption after intramuscular administration, and direct interference with its enzymatic activity.11
Currently, there are no commercially available, clinically validated assays for IgG or IgE anti-l-asparaginase antibodies. Moreover, the specificity of anti-l-asparaginase antibodies to predict inactivation has been low in comparison to measuring l-asparaginase activity itself; many patients develop anti-l-asparaginase antibodies without clinical allergic reactions or inactivation of the enzyme, and antibody levels in children with and without hypersensitivity overlap.12
Importantly, no correlation has been found between IgG antibody titers and the severity of the allergic reaction.13 This is probably because IgG anti-l-asparaginase antibody assays are used as a surrogate for the diagnosis of l-asparaginase allergy, and non-allergic ALL children can develop specific IgG anti-l-asparaginase antibodies, rendering its diagnostic utility controversial.14 Specific IgE anti-l-asparaginase antibodies, on the other hand, contribute to clinical symptoms through mediator release from mast cells.15 Thus, controversy on the meaning of anti-l-asparaginase antibodies remains although its prognostic significance and clinical utility has been studied for over 30 years.16 Several important questions remain, including what is the association between IgE anti-l-asparaginase antibodies and ALL clinical events other than allergic reactions. Furthermore, the time during which IgG and IgE antibodies can be detected has not been established.
This study investigated the production of IgG and IgE anti-l-asparaginase antibodies in children diagnosed with B cell ALL treated with a standardized dose of E. colil-asparaginase and determined the association of these two antibodies with the clinical course and risk of relapse.
MethodsA transversal descriptive cross-sectional study was conducted in the Hematology, Allergy, and Immunology Departments, of the “José Eleuterio González” University Hospital of the Universidad Autónoma de Nuevo León, Monterrey, Mexico. Under 16-year-old patients with diagnosis of B-cell ALL confirmed by flow cytometry at any stage of treatment after induction to remission therapy were included. Children taking anti-H1 or anti-H2 antihistamines were excluded. The study was approved by the Institutional Review Board and Ethics Committee of the institution and parents signed informed consent forms.
Induction to remission therapy consisted of prednisone 60mg/m2, vincristine 1.5mg/m2, and six doses of l-asparaginase of 6000IU/M2/intramuscular on Days 8, 12, 16, 20, 24, and 36. Children with high-risk ALL received two additional doses of l-asparaginase on Days 2 and 8 of re-induction and three doses of doxorubicin (40mg/m2); triple intrathecal chemotherapy for central nervous system (CNS) prophylaxis was administered four times. Consolidation included single doses of cytosine arabinoside (1.5g/m2) and methotrexate (1.5g/m2) administered in a one-day intravenous infusion. This was followed by one month of 6-mercaptopurine taken daily and weekly methotrexate. Re-induction included 15 days of prednisone, three doses of vincristine, two of doxorubicin for high-risk and one for standard-risk patients, two doses of l-asparaginase and two of triple intrathecal prophylaxis. Ten days after re-induction, maintenance was started for 90 weeks with oral 6-mercapthopurine at 50mg/m2/day and weekly methotrexate starting at 30mg/m2/day and adjusted to maintain the absolute leukocyte count between 3.0 and 5.0×103/μL. Every six weeks during the first year of maintenance, and every three months during the second year, maintenance was suspended for a week in order to administrate one single dose of vincristine, triple intrathecal chemotherapy, and seven days of prednisone; this regimen was inspired on a previously published protocol.17 Prophylaxis of the CNS consisted in triple intrathecal therapy including cytosine arabinoside, methotrexate, and hydrocortisone. CNS irradiation was reserved for patients with infiltration at diagnosis and those suffering CNS relapse. Relapsed children received additional l-asparaginase if they did not develop clinically evident hypersensitivity manifestations.
Immunoglobulin G anti-l-asparaginase antibody determinationIgG anti-l-asparaginase serum antibodies were determined employing an enzyme-linked immunosorbent assay (ELISA) following a previously reported method.18 Briefly, peripheral blood collected from patients and ten healthy controls was centrifuged for five minutes at 3000rpm and the separated serum was stored at −70°C. Leunase, 10,000IU (Kioto, Japan) was diluted in 0.05M carbonate-bicarbonate buffer (pH 9.4–5μg/mL); 100μL of this dilution were added, in duplicate, to 96-well polystyrene ELISA plates (WWR Scientific Product, GA, USA) followed by overnight incubation at 4°C. The supernatant was discarded and the plates were washed with phosphate buffered saline (PBS) containing Tween-20, 0.1%. Phosphate buffered saline (300L), containing 0.5% bovine serum albumin (BSA) (Sigma, St. Louis, MO, USA), 5% fetal bovine serum and 0.1% Tween-20, was added to each well, followed by an incubation of 90min at room temperature (RT); excess supernatant was discarded and the wells were washed three times with the PBS/tween-20 solution. For the assay, 100μL of plasma of patients and controls, diluted 1:3200 in saline-tween were added in duplicate to a 96-well polystyrene plate to which l-asparaginase was previously attached, as described above,18 including negative and positive controls. As no severe or anaphylactic reactions to l-asparaginase developed in the children of this study, positive IgG anti-l-asparaginase control serum was obtained from sensitized mice. Briefly, 15mg of Leunase (Kyowa Hakko Kogyo Co., Japan) in incomplete Freund adjuvant solution was injected into the peritoneum of Balb/c mice between six and eight weeks of age. Additional immunizations were given on Days 15 and 30 injecting 10mg of the enzyme in the same Freund solution. On Day 35 post-immunization, blood was taken from the retro-orbital vascular plexus and centrifuged at 3000rpm; finally, mice sera were pooled and tested; the mixed serum with the highest IgG anti-l-asparaginase titer, as assayed above, was the positive control. Negative controls consisted of sera from non-immunized mice, normal human sera, and diluent alone. Plates were incubated with continuous agitation at 37°C for one hour; the supernatant was discarded and the wells washed three times with saline-Tween-20; 150μL of a secondary peroxidase-conjugated monoclonal goat anti-human IgG antibody (Sigma, St. Louis, MO, USA) was added to each well and incubated at 37°C for one hour. Four washes were followed by the addition to each well of 100μL of a substrate-chromogen solution containing o-phenylenediamine dihydrochloride (OPD):2HCl (Sigma, St. Louis, MO, USA), hydrogen peroxide, and citrate buffer, then incubated for 30min at room temperature in the dark. The reaction was stopped by adding 100μL of a 1.0M phosphoric acid solution; l-asparaginase antibodies were expressed as optical density (OD) readings. Samples were defined as positive if the natural log of the 1:3200 OD reading was greater than two standard deviations above the negative control processed mean.19
Immunoglobulin E anti-l-asparaginase antibody detectionDue to the lack of a commercially available standardized assay for IgE anti-l-asparaginase antibodies, we decided to assess IgE-mediated type I hypersensitivity reaction to l-asparaginase in vivo. Thus, two types of validated skin testing were performed, a prick skin test (PST)20 and intradermal skin test (IST).21 The PST was carried out by applying one drop of a 30μM l-asparaginase solution (Leunase, Kyowa Hakko Kogyo Co., Japan) followed by a skin puncture using a disposable plastic lancet (Duotip® by Lincoln Diagnostic, Inc., Decatur, IL). The solution was left in contact with the skin for 15min. Afterwards the papule and erythema were observed and the diameter was measured in millimeters by the same experienced allergist in all cases using a millimeter scale. The IST was performed by injecting 10μL of the same sterile l-asparaginase solution; after 15min, the papule and erythema were measured in millimeters in the same way as for the PST. An intradermal injection of 10μL of 1% histamine phosphate solution was the positive control for the skin test; normal saline solution was the negative control. A wheal of 3mm or larger than the negative control was interpreted as a positive test.20
Statistical analysisThe Chi-square test was used to analyze frequencies, the Mann–Whitney test for independent variables with non-normal distribution, and Spearman's method were used to study IgG–IgE correlations. The Kaplan–Meier method was used to compare event-free survival (EFS) between children who did and did not have IgG antibodies, and between those with and without an IgE response to l-asparaginase as evaluated using the PST and IST. The Statistical Package for the Social Sciences (SPSS – v20) was employed.
ResultsFifty-one patients were studied; pertinent descriptive data are shown in Table 1. Children received a median of eight doses (range: 5–14) of l-asparaginase. According to the clinical files and electronic records, only three (5.8%) patients suffered any allergic/hypersensitivity reaction consisting of urticaria/skin rash after receiving 8, 8, and 13 l-asparaginase doses, respectively; there were no anaphylactic reactions.
Important characteristics of 51 children with acute lymphoblastic leukemia.
| Characteristic | |
|---|---|
| Age, years – median (range) | 8 (4–17) |
| Gender – n (%) | |
| Male | 27 (53) |
| Female | 24 (47) |
| Risk group – n (%) | |
| Standard-risk | 23 (45.1) |
| High-risk | 28 (54.9) |
| Clinical status at the time of the study – n (%) | |
| Maintenance (≥1 year) | 27 (52.9) |
| Surveillance | 16 (31.3) |
| Relapse | 8 (15.6) |
| Anti-l-asparaginase antibodies – n (%) | |
| IgG (ELISA) | 24 (47.0) |
| IgE (in vivo) | 18 (35.3) |
| None | 9 (17.7) |
In total 42/51 (82.35%) patients had IgG, IgE, or both anti-l-asparaginase antibodies; ten (23.8%) had exclusively IgG antibodies, whereas 18 (42.86%) had exclusively IgE antibodies; of the forty-two children with antibodies, IgG was present in 24 (57.14%), and IgE in 32 (76.19%); 14/42 (33.33%) developed both IgG plus IgE antibodies. No IgG or IgE antibodies were found in 9/51 (17.64%) children.
With regard to associations between risk group and antibodies, 19/23 (82.6%) standard-risk patients developed antibodies compared to 23/28 (81.2%) high-risk children (p-value>0.05). When antibody distribution was studied it was found that six (21.4%) high-risk patients developed IgG antibodies, compared to four (17.4%) in the standard-risk group, while IgE antibodies were detected in 13 (46.4%) vs. five (21.7%), and IgG together with IgE antibodies in four (14.3%) vs. ten (43.46%) high-risk and standard-risk children, respectively (p-value=0.38).
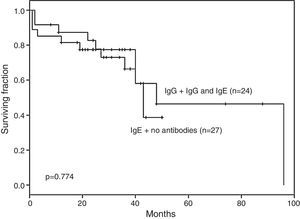
No difference was documented in EFS for ALL children with IgG and IgG plus IgE anti-l-asparaginase antibodies (n=24) vs. those with no antibodies or with IgE alone (n=27 – p-value=0.774; Figure 1).
Neither the presence nor the titer of IgG anti-l-asparaginase antibodies influenced the response to induction or re-induction to remission therapy (p-value=0.19). Furthermore, no difference in response to ALL induction or re-induction therapy was documented between children with a positive or a negative skin test (p-value=0.82). Table 2 compares the type and prevalence of antibodies in the current study sample with the results of reports in the literature.
Anti-l-asparaginase antibodies identified in this study and in representative publications.
| Reference | n | IgG | IgE | IgG+IgE | IgM | None |
|---|---|---|---|---|---|---|
| Current study | 51 | 10 (19.6%) | 18 (35.3%) | 14 (27.5%) | 9 (17.6%) | |
| Panosyan et al.1 | 1001 | 611 (61.0%) | 390 (39.0%) | |||
| Zalewska-Szewczyk et al.8 | 13 | 5 (38.5%) | 8 (61.53%) | |||
| Woo et al.13 | 152 | 54 (35.5%) | 98 (64.5%) | |||
| Zalewska-Szewczyk et al.14 | 47 | 20 (42.5%) | 19 (40.4%) | 8 (17.0%) | ||
| Cheung et al.16 | 13 | 7 (53.8%) | 6 (46.2%) | |||
| Kawedia et al.19 | 35 | 28 (80.0%) | 7 (20.0%) |
Ig: Immunoglobulin.
Prick skin testing was performed in all 51 children with 28 (54.9%) having positive results; 23 (45.1%) patients with negative PST were submitted to IST with only four having positive results. Thus, 32 (62.7%) of 51 children had specific IgE anti-l-asparaginase antibodies demonstrated by a positive skin test in vivo.
Eighteen (35.3%) patients of the whole group relapsed, two (8.7%) of the 23 standard-risk children and 16 (57.1%) in the 28 high-risk group (p-value<0.001).
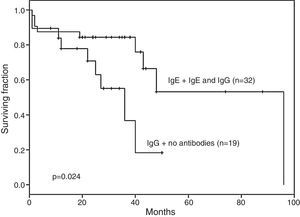
The EFS of the 19 patients who developed IgG anti-l-asparaginase antibodies only and those with no antibodies was significantly lower than in the remaining 32 patients, [36 months (range: 27–40) vs. 96 months (range: 62–99); p-value=0.024 – Figure 2].
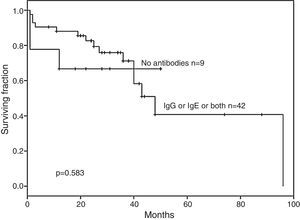
No statistically significant difference in EFS was documented for children with no antibodies (n=9) against l-asparaginase compared to those having IgG, IgE or both (n=42) antibodies (p-value=0.583 – Figure 3).
Eighteen (35.3%) patients relapsed at a median of 20.5 months (range: 1–96), ten relapsed during first maintenance, five after cessation of therapy, and three several years after completing treatment. Nine (50%) had exclusively IgG anti-l-asparaginase antibodies and nine (50%) had positive skin tests indicating the presence of IgE anti-l-asparaginase antibodies, none had both. Fifty-five percent (5/9) of the children who relapsed who had IgG, but not IgE detectable by skin testing, suffered additional relapses (range: 2–4). In contrast, none of the nine patients who relapsed and had a positive skin test suffered additional relapses (p-value<0.01). Thus, patients with negative skin tests had a multiple-relapse hazard ratio of 2.87 (95% confidence interval: 1.041–7.944; p-value=0.042).
DiscussionThe relevance of antibodies directed against l-asparaginase in children with ALL has been consistently highlighted in the Berlin–Frankfurt–Münster (BFM) studies and recently reviewed.8,12 Adverse reactions to the enzyme can be mediated by IgG, complement or IgE antibodies, or more than one at the same time.22 A neutralizing nature of IgG anti-l-asparaginase antibodies leading to accelerated plasmatic clearance, with an important decrease in l-asparaginase activity, has been reported23; the titer of these antibodies can increase considerably after switching to pegylated l-asparaginase following the development of hypersensitivity.24 Interestingly, the different roles of these anti-l-asparaginase antibody classes and their correlation with clinical allergy manifestations and the development of silent antibodies has not been discussed.
Allergic reactions are associated with the appearance of antibodies, which have been reported to increase l-asparaginase clearance and to reduce or even neutralize the catalytic activity of the enzyme.14,25 ALL patients who develop anti-l-asparaginase antibodies may thus have poor outcomes because of low l-asparaginase activity.14,19
A report on the diagnostic utility of serum antibody testing in 410 children with ALL receiving l-asparaginase found that 169 (41.2%) had some degree of clinical allergy and 148 (87%) had IgG anti-l-asparaginase antibodies; of the remaining 241 patients with no allergy, 89 (36.9%) had IgG antibodies. IgE antibodies were not determined.26
The goal of the current study was to investigate the presence of both IgG and IgE antibodies to l-asparaginase and to explore their clinical correlation with the course of the disease. Over 80% of children developed antibodies, almost 60% of these were IgG and only these were associated to lower EFS most probably due to their l-asparaginase neutralizing nature; the incidence of anti-l-asparaginase antibodies in this study sample (82.35%) was similar to the 87% reported in a large prospective study.27 Remarkably, IgG and IgE antibodies were detected in some cases several years after the patient's last exposure to the enzyme thus confirming that l-asparaginase is strongly immunogenic and that immune memory mechanisms are active and operational during the evolution and therapy of ALL. It is important to point out that current recommendations state that anti-asparaginase antibody and asparagine measurements are not indicated for clinical decision making outside the context of clinical trials.12
Furthermore, it is important to underscore that there are patients with decreased serum l-asparaginase activity and no demonstrable IgG antibodies to the enzyme, suggesting that additional factors are involved in this phenomenon. These factors include the number of doses and intensity of the l-asparaginase regimen, concomitant administration of strong immunosuppressive drugs as part of the chemotherapeutic regimen and enhanced enzyme clearance associated to protease degradation.
It is noticeable that given the frequency and severity of reactions to l-asparaginase in children with ALL, few studies have analyzed the correlation of IgE with clinical events. The current study found a prevalence for positive skin tests of 63% (32/51) employing the PST and IST, although only three of these children (9.4%) developed hypersensitivity reactions, suggesting that production of IgE anti-l-asparaginase antibodies mostly occurs silently.28 In this respect, allergen-specific IgE antibodies are responsible for sensitizing mast cells and recognizing allergens in immediate hypersensitivity reactions. IgE binds to eosinophils, basophils and mast cells. After re-exposure, mast cells proliferate and release histamine, prostaglandin and cytokines, which mediate clinical hypersensitivity symptoms. The low frequency of allergic reactions in the children of this study could be related to the routine practice in our institution of pre-medication with anti-histamines to prevent allergic reactions to the enzyme, the low-dose l-asparaginase regimen used (6000IU/M2 compared to 10,000IU/M2 in other reports)8,13,21 and the lower median number of l-asparaginase doses [8 (range: 5–15) compared to 13.5 (range: 4–20) in other reports]. Both dose and number of doses are directly related to the development of hypersensitivity events.3 Interestingly, despite the fact that high-risk ALL children received more l-asparaginase doses than standard-risk patients (9 vs. 6), there was no difference in the rate of anti-l-asparaginase antibodies produced. We hypothesize that this could be due to the existence of a critical threshold of immunizing events to l-asparaginase, beyond which l-asparaginase-non-responders remain antibody-free. Genetic factors may influence the likelihood of developing clinical hypersensitivity reactions; using a genome-wide approach, it was reported that genetic variations in GRIAI were associated with asparaginase allergies.29
The basophil activation test (BAT), in which the surface expression of the degranulation/activation marker (CD203c) on basophils is detected, is considered a reliable tool for diagnosing IgE-mediated allergies. Recently, it has been shown that BAT is a useful marker for identifying l-asparaginase allergy because of its high sensitivity and specificity, and combining the BAT with an l-asparaginase-specific IgG assay is the most accurate method of identifying l-asparaginase allergy.30
This study found a positive correlation between the presence of IgG anti-l-asparaginase antibodies and ALL relapse, confirming previous studies that found a negative influence of neutralizing IgG antibodies, manifested as lower EFS and overall survival.1,3,15 Remarkably, the presence of IgE antibodies documented by skin testing was not associated to a higher relapse rate. This is probably because this class of antibodies lacks neutralizing activity; on the contrary, there was an association between negative skin tests and elevated risk of additional relapses. Thus, children who suffered a first relapse and had negative skin tests had a statistically significant increased risk of subsequent relapses: 55% compared to 0% in those with positive skin tests (p-value<0.0001). We hypothesize that children with more than one relapse and negative skin tests might have additional defective immune surveillance and blunting of the critical process of host-dependent secondary elimination of the residual leukemic clone, thereby favoring ALL relapses. In this respect, both, humoral and cellular immune functions are decreased during ALL and its therapy31; regaining immune function can take several years to accomplish, and even then, the recovery of IgG subclasses can be impaired.32 Additionally, relapsed children with IgE antibodies may have a limited capacity to produce IgG neutralizing antibodies and then have a lower risk of relapse.
The hypothesis that children with IgG anti-l-asparaginase antibodies, in isolation or associated with IgE antibodies, would clear the drug from the blood and children would relapse more frequently than those without IgG antibodies was not confirmed by Kaplan–Meier analysis (p-value=0.774). In addition, the hypothesis that IgE anti-l-asparaginase antibodies, alone or in combination with IgG antibodies, would be detrimental because children who developed IgE antibodies would not receive the same amount of l-asparaginase due to supervening allergic reactions was not confirmed. On the contrary, IgE seemed to protect children with this antibody, alone or in combination with IgG antibodies (p-value=0.024). Accordingly, IgE might play a dual role in ALL, as a negative factor due to its participation in hypersensitivity reactions, forcing the cessation of therapy and the switching of enzyme preparations, or as a positive surrogate indicator for residual immune competence and less additional relapses. Moreover, there is the possibility of some interaction, either positive or negative, between the effects of IgG and IgE antibodies. These results appear equivocal, which may be due to the small number of children in each group; the literature however, does not offer a definitive answer in this respect yet and interestingly, there are no studies documenting a link between the presence of IgE anti-l-asparaginase antibodies and decreased serum activity of l-asparaginase. The last consensus recommendations consider that the development of either antibody is detrimental to children with ALL.12 However, when the current small group was analyzed, no significant difference was found, although the comparison showed a higher EFS, albeit non-significant (p-value=0.583), in the no-antibodies group (n=9; median 68 months) vs. those with any type of antibody (n=42; median 45 months). These results suggest that there are different implications according to the class of antibody present. IgG antibodies are associated to a bad prognosis and IgE has either a negative association due to hypersensitivity reactions, or a positive association conferring resistance to subsequent relapses, probably as a surrogate indicator of residual immune competence in children, leading to final clearance of the leukemic clone.
Limitations in our proof-of-concept study include the small sample size and its retrospective design. Additionally, the relapse rate was higher than expected, reflecting the fact that most patients referred to our center have unfavorable clinical and hematologic characteristics at diagnosis and were treated with a low-moderate dose intensity protocol, as well as the known greater incidence of high-risk children in the Hispanic population.33 Another major limitation is the heterogeneity of the clinical stages at the time of the single determination of IgG and IgE antibodies for this cross-sectional, proof-of-concept study, and thus in order to confirm these findings a prospective, sufficiently powered study, including balanced groups at all major time points of treatment, is required.
In conclusion, children with only IgG antibodies against l-asparaginase suffered more relapses than those without these antibodies or when IgE was simultaneously present and patients with IgE positive skin tests for the enzyme had a decreased risk of suffering more than one relapse. Forty-five years after the initial report,34 critical aspects of the immune response to l-asparaginase in ALL are still undefined; prospective studies aimed at deciphering the intricate nature of this response mediated by IgG and IgE antibodies are necessary to definitively establish their interactions and influence on the outcomes of ALL of childhood.
Conflicts of interestThe authors declare no conflicts of interest.