Chronic lymphocytic leukemia is characterized by clonal proliferation and progressive accumulation of B-cell lymphocytes that typically express CD19+, CD5+ and CD23+. The lymphocytes usually infiltrate the bone marrow, peripheral blood, lymph nodes, and spleen. The diagnosis is established by immunophenotyping circulating B-lymphocytes, and prognosis is defined by two staging systems (Rai and Binet) established by physical examination and blood counts, as well as by several biological and genetic markers. In this update, we present the recommendations from the Brazilian Group of Chronic Lymphocytic Leukemia for the diagnosis and treatment of chronic lymphocytic leukemia. The following recommendations are based on an extensive literature review with the aim of contributing to more uniform patient care in Brazil and possibly in other countries with a similar social–economic profile.
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in adults and accounts for approximately 30% of all leukemias in this population group. The annual incidence of CLL in the United States is approximately 4.6 cases/100,000 persons per year. The median age at diagnosis is 71 years, and over 95% of patients are older than 50 years.1 CLL is less frequent in individuals with Asian and Middle Eastern ancestry.2 It is slightly more common in males, with a 1.25:1 male:female ratio.3
The etiology of CLL is still unknown. Genetic and environmental factors may have an important role. The low frequency of CLL in individuals with Eastern ethnicity and the higher incidence in family members (5–10%) than other mature B-cell neoplasms reflect the potential importance of a genetic factor.4 CLL used to be considered a disease of naïve B-cell lymphocytes however recent studies suggest there is a post-germinal center origin.5
The clinical presentation at diagnosis is extremely variable. Approximately 60% of patients are asymptomatic, and the disease may be suspected after a routine blood count. When symptomatic, patients present with vague symptoms of fatigue or weakness.6
Patients usually have a good performance status at diagnosis. Lymphadenopathy may be observed in approximately 80% of cases often with cervical and axillary lymph nodes bilaterally and symmetrically being affected. Splenomegaly is usually mild to moderate and is observed in approximately 50% of cases; hepatomegaly is less frequent.7,8 Although rare at diagnosis, as the disease progresses patients can have B symptoms, which are defined as unintentional weight loss of 10% or more within six month, fever above 38°C for two or more weeks without other evidence of infection, and night sweats for more than a month without evidence of infection.
Anemia and thrombocytopenia may be observed in 15–30% of patients. They generally result from bone marrow infiltration, although they can also be related to an autoimmune phenomenon [autoimmune hemolytic anemia (AIHA), immune thrombocytopenia (ITP), and immune neutropenia].7,8 Lymphocytosis is always present, but the absolute number of lymphocytes is extremely variable. In a recent analysis by the Brazilian CLL Registry (unpublished data), the median hemoglobin level was 13g/dL, platelet count was 180×109/L, white blood cell count was 35×109/L (range: 7–900×109/L), and lymphocyte count was 27×109/L (range: 5.4–891.0×109/L) among 1612 Brazilian patients with CLL.
Richter Syndrome, which is defined as the transformation of CLL into an aggressive lymphoma (most commonly diffuse large B-cell lymphoma) occurs in 5–10% of all cases. The syndrome may be suspected if there are signs of aggressive disease, such as impairment of performance status, presence of B symptoms, and rapid increase in the size of lymph nodes.1
Infections are common complications of CLL due to the deficiency of both the cellular and humoral immune system. T cells, natural killer cells, neutrophils, and monocytes/macrophages may be significantly compromised.9,10 Furthermore, hypogammaglobulinemia is not rare and can become more intense after CLL treatment.11 Although preventive use of intravenous immunoglobulin is controversial, it may be necessary if there are severe recurrent infections.12,13 Bacterial infections are common even prior to the treatment of CLL. The most common agents are Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. Response to immunization is variable, and vaccination should be carried out early in the disease to obtain the best results. Live virus vaccines should be avoided.14 Viral infections can also occur, and special attention should be paid to herpes zoster reactivation. Fungal infections or opportunistic bacteria, however, are rare in untreated CLL. The introduction of immunosuppressive drugs significantly increases the risk for cytomegalovirus infections, as well as Pneumocystis jiroveci, Listeria monocytogenes, and fungal infections.14
Autoimmune complications can occur during the disease course,15–17 and the most common complication is AIHA (occurs in approximately 3% of patients with stable disease). The incidence of AIHA increases with disease progression (up to 11% in late-stage Binet B and C), and up to 15% of CLL patients may have positive direct antiglobulin (DAT) or Coombs tests during the disease course, including non-anemic patients.18 The diagnosis of AIHA may be difficult because the reticulocyte count can be low due to erythroid hypoplasia when bone marrow is extensively infiltrated by CLL. Increased Lactate dehydrogenase (LDH) levels may occur as the disease progresses, and other associated factors, such as impaired hepatic function and bilirubin, may be preserved with normal liver function. Measurement of serum haptoglobin may be useful in this setting.17 Other immune cytopenias can occur less frequently. Clinically significant immune thrombocytopenia (2% of CLL patients) should be suspected when there is a rapid drop in platelets and no evidence of bone marrow failure.18 Approximately one third of cases can evolve to Evans syndrome. There is usually a good response to first-line therapy (steroids or intravenous immunoglobulin), but approximately 20% of cases are refractory. These patients may benefit from rituximab alone or in association with cyclophosphamide and dexamethasone. Splenectomy should be reserved for select refractory cases.19 Pure red cell aplasia and autoimmune neutropenia can also occur, but these conditions are extremely rare.18
Secondary malignancies, such as skin and lung cancer, are more common in CLL patients than in the general population, which may be due to immunodeficiency.20
DiagnosisCLL is defined by the presence of at least 5×109/L CD5+/CD23+ monoclonal B lymphocytes in peripheral blood (PB) for more than three months,21 with immunophenotyping of PB being sufficient for diagnosis. Small lymphocytic lymphoma is distinguishable only by its non-leukemic appearance and requires the presence of lymphadenopathy and/or splenomegaly and a clonal B lymphocyte count (CLL immunophenotype) that does not exceed 5×109/L in the PB.22
CLL-type monoclonal B lymphocytosis (MBL) is defined by the presence of fewer than 5×109/L B-cells with the CLL phenotype in the PB in the absence of lymphadenopathy, spleen or hepatic enlargement, cytopenias, and disease-related symptoms.23 In the 2016 revision of the WHO classification of lymphoid neoplasms, a distinction between low-count (<0.5×109/L) and high-count MBL (>0.5×109/L) was recommended because low-count MBL has a low probability of progressing to CLL, while high-count MBL may progress at a rate of 1–2% per year.24,25 The incidence of MBL among a healthy population may vary depending on the sensitivity of the diagnostic method but can reach up to 12%.26
Morphological evaluation of a blood smear should show small mature lymphocytes with a narrow cytoplasm, a dense nucleus with partially aggregated chromatin, and the absence of visible nucleoli. The percentage of prolymphocytes in blood lymphocytes may be <55%. A higher percentage would favor the diagnosis of prolymphocytic leukemia. Gumprecht shadows are frequently found on CLL PB slides.22
CLL lymphocytes exhibit a characteristic profile of CD19+, CD5+, CD23+, CD20+ low, CD200+, CD22+ low/negative, CD79b+ low/negative, CD43+ low, sIgκ+ or sIgλ+ low, sIgM+ low, CD11c+ low/negative, FMC7 negative, CD10 negative, and CD103 negative.
Matutes et al.27 proposed a scoring system for the diagnosis of CLL (modified in 1997)28 based on the evaluation of five parameters: CD5+ (1 point), CD23+ (1 point), FMC7 negative (1 point), weak intensity of kappa/lambda chains (1 point), and weak or negative CD22/CD79b (1 point). The CLL score ranges between 5 (typical CLL cases) and 3 (less typical CLL cases). Scores of 0–2 exclude the diagnosis of CLL.
Classical laboratory markers for adverse prognoses of CLL are CD38,29 CD49d,30 and ZAP-70.31 The cut-off values for CD49d and CD38 are at least 30%, while the cut-off for ZAP-70 is not clear and technically controversial.32 The expressions of some markers, such as CD305 (LAIR-1), CCR6, and CXCR5 are also associated with some high-risk chromosomal abnormalities.33
Recommendations of the Brazilian Group of CLL group for diagnosis- 1)
Complete blood count and differential white blood cell count;
- 2)
Morphological evaluation of a blood smear;
- 3)
PB immunophenotyping: at least a 4-color panel of the fluorochromes FITC, PE, PerCP or PerCPCy5.5, and APC performed according to the consensus of the Brazilian Group of Flow Cytometry (GBCFLUX) for Chronic Lymphoproliferative Disorders (in press). This panel should include one screening tube (CD8/Lambda, CD56/Kappa, CD19/CD4, CD3) and two diagnostic tubes, including CD45, CD20, CD19, CD5, CD79b, CD23, CD19, and CD200. The Brazilian Group of CLL also recommends two additional tubes for prognostic and minimal residual disease (MRD) purposes, including CD38, CD305, CD19, CD49d, CD81, CD43, CD19, and sIgM. ZAP-70 is considered an optional prognostic marker.
Panels using six, eight, or more colors should use the same markers in appropriate combinations. The Euroflow group34 designed an 8-color panel of monoclonal antibodies (MoAbs) to improve diagnosis and to differentiate CLL from other B cell lymphoproliferative disorders. They included all MoAbs proposed by Matutes with the exception of FMC7 and CD22, and they incorporated other markers such as CD43, CD81, CD38, CD20, and CD200. CD200 is particularly useful for differentiating CLL from mantle cell lymphoma.
- 4)
Bone marrow biopsy and aspiration are NOT recommended for routine CLL diagnosis. This procedure may be performed in patients who will participate in clinical trials and/or patients with persistent cytopenias after treatment to differentiate leukemic infiltration from therapy-related toxicity. The bone marrow smear should be characterized by the presence of CLL cells, the percentage of which is typically above 30%. Infiltration in a bone marrow biopsy may have a nodular, interstitial, or diffuse growth pattern.
- 5)
Imaging techniques, such as ultrasound, computed tomography, magnetic resonance imaging, and positron emission tomography-CT (PET-CT) scanning, are NOT recommended for routine CLL diagnosis or staging.
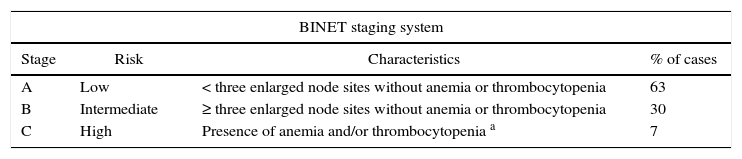
Two clinical staging systems (Rai and Binet) were introduced in the 1970s and are still widely used. They are easy to apply in the clinical practice, based only on clinical data, and take into account lymph node, spleen, and liver involvement, as well as the presence of cytopenias (anemia and thrombocytopenia) (Table 1). Some patients in the low-risk group (0–1 Rai and Binet A) may, however, exhibit rapid disease progression, while others remain in a stable disease condition for many years.7,8 Other prognostic factors have been researched in an attempt to predict disease evolution by taking into account other clinical and biological prognostic factors. Unfavorable prognostic factors include male gender, an initial white blood cell count above 35×109/L, lymphocyte doubling time (LDT) of less than six months, a diffuse histological pattern in bone marrow infiltration35 and elevated levels of beta-2 microglobulin, LDH, serum thymidine, and serum CD23 at diagnosis.36 As previously discussed, high expressions of CD38,29 CD49d,37 CD305,38 and ZAP-70 protein detected by flow cytometry are also prognostic markers.31 However, these markers are no more sensitive than clinical staging for determining tumor burden and predicting disease progression.
Clinical staging and survival.110
| BINET staging system | |||
|---|---|---|---|
| Stage | Risk | Characteristics | % of cases |
| A | Low | < three enlarged node sites without anemia or thrombocytopenia | 63 |
| B | Intermediate | ≥ three enlarged node sites without anemia or thrombocytopenia | 30 |
| C | High | Presence of anemia and/or thrombocytopenia a | 7 |
Immunoglobulin heavy chain variable region (IGHV) mutation status has an important role in CLL prognosis. Mutated IGHV is related to an indolent clinical course and a ‘non-mutated’ state with a more aggressive disease progression.39,40 However, determining IGHV mutation status involves expensive and labor-intensive molecular techniques, which has limited its use in clinical practice.
Chromosomal abnormalities have an important role in establishing CLL prognosis. Whenever possible, a G-banding karyotype should be performed because patients with complex aberrations often present unfavorable outcomes. Given the difficulty in obtaining abnormal metaphases, fluorescence in situ hybridization (FISH) is more efficient for finding major genetic abnormalities in CLL. Using FISH, cytogenetic changes have been found in 80% of cases41; trisomy 12 was reported in 10–20% of cases, deletion of 13q [del(13q14.1)] was reported in approximately 55% of cases, deletion of 11q [del(11q22-23)] was reported in 10–25% of cases, and deletion of 17p [del(17p)] (P53 locus) was reported in 5–10% of cases.42 While del(13q14.1) is related to a more favorable prognosis, the association between trisomy 12 and prognosis is still not clearly defined,32 and del(11q22-23) associated with bulky disease is related to a more unfavorable outcome. Chemoimmunotherapy with purine analogs seems to overcome the del(11q22-23) prognostic effect.41,43
In approximately 7% of cases, del(17p) is found at diagnosis. It is a cytogenetic aberration associated with the worst CLL prognosis and has led clinicians to change their first-line treatment.41 However, not all patients with del(17p13) require therapy at the time of diagnosis. Only approximately 50% of treatment-naïve CLL patients with del(17p13) developed progressive disease that required therapy within 12–18 months, while the other half had a relatively stable disease extending out to 70 months of follow-up.44
Using Sanger sequencing, several studies reported that monoallelic mutations in TP53 are associated with poor prognosis in CLL and resistance to standard therapy.45,46TP53 disruption (either by mutation or deletion) is present in approximately 15% of patients, and some have both the TP53 mutation and del(17p13), as detected by FISH. Some have no TP53 mutation but have del(17p13) based on FISH analysis, and some (3–5%) have the TP53 mutation but no del(17p13) based on FISH. Importantly, these patients had a short OS that was comparable to the OS of patients with del(17p13) based on interphase FISH.46–48 Less than 10% of patients present with a TP53 mutation at diagnosis, while the mutation is present in up to 50% of cases in pretreated cohorts, including cases of Richter's transformation.49
Clone size according to FISH is also extremely relevant to CLL. As recently shown, patients with ≤20% 17p deletion nuclei had a longer median time to first treatment (TTFT) and beffer overall survival (OS) from the date of the first FISH study (44 months and 11 years, respectively) and were more likely to have an IGHV mutation.50
Despite the lack of disease-defining molecular alterations in CLL, some recurrent somatic gene mutations, including mutations in TP53, ATM, NOTCH1, SF3B1, BIRC3 genes, and others, have been described as important prognostic markers and are potential therapeutic targets. However, only TP53 has been consistently described as a clear high-risk marker of therapy refractoriness and early relapse to date, and patients could benefit from different treatment approaches.25
More recently, the German CLL Study Group proposed the CLL International Prognostic Index IPI (CLL-IPI), which combines the most important genetic risk factors (IGHV, del(17p)/TP53 mutations) with clinical stage, age, and beta-2 microglobulin level.51
Recommendations of the Brazilian Group of CLL for prognosis stratificationThe recommendations are to investigate the del(17p13) detection with FISH and test for the TP53 mutation before initiating first-line treatment. Whenever possible, testing for the most frequent genetic aberrations, such as del(13q), del(11q), +12, IGHV mutation, and G-banding karyotype, should be performed as well. Moreover, whenever possible during disease course, the TP53 mutation and 17p deletion should be investigated by FISH before initiating a new treatment because there may be clonal selection after the first treatment that may require a change in the treatment paradigm.
Overview of minimal residual diseaseMinimal residual disease (MRD) evaluation after three and six cycles of therapy regimens and three months after the end of treatment seems to be an important outcome predictor for CLL treatment, and it has been increasingly used in conjunction with the more traditional endpoints of progression-free survival (PFS) and OS.52,53
MRD is also an important predictor of outcome after hematopoietic stem cell transplantation (HSCT). MRD-negative status 12 months after HSCT has a high prognostic significance.54 However, this time point must be validated in prospective clinical trials.
Both immunoglobulin heavy chain (IgH)-PCR and flow cytometry can be used to asses MRD. In the European Research Initiative on CLL (ERIC) study, the flow cytometry approach was identified and validated to be reliable in clinical trials and the clinical practice. The use of the MoAb CD19/CD5/kappa/lambda combination may be important for identifying cases that do not require extensive analysis for MRD detection. These cases must have a CD19+ cell percentage >9% of total leukocytes, a CD19+ κ:λ ratio <0.04:1 or >61:1, and in cases with sufficient CD5+ B cells for enumeration, >82% of B cells co-expressing a CD19+CD5+ κ:λ ratio of <0.05:1 or >32:1 or >54% of CD19+CD5+ cells lacking surface immunoglobulin.55
The initial ERIC recommendations were based on a 4-color panel of monoclonal antibodies (FITC/PE/PerCP/APC): CD20/CD38/CD19/CD5; CD81/CD22/CD19/CD5 and CD79b/CD43/CD19/CD5, with a component specification independent of instrument and reagents at the 0.01% (10−4) level.55
In an effort to quantify CLL cells at a level of 0.001% (10−5), another ERIC assay was proposed with six markers (FITC/PE/PerC5.5/PE-Cy7/APC/APC-H7): CD3/CD38/CD5/CD19/CD79b/CD20 and CD81/CD22/CD5/CD19/CD43/CD20).
Interestingly, this approach correlated well with the 4-color panel, with good linearity even at 0.001% (10−5) sensitivity. Recently, the ERIC project validated a reliable approach to evaluate MRD in CLL to a level of 10−5 using an 8-color tube, with a component specification independent of equipment and reagents. They identified markers that have a substantial impact on the ability to detect MRD and composed a 6-color core panel (i.e. CD19, CD20, CD5, CD43, CD79b, and CD81), incorporating CD200 or CD 23 to simplify the diagnosis, or also testing alternative CLL MRD markers such as CD160 or ROR1, which was shown to be a very useful marker to discriminate normal B cells from CLL cells.56
Raponi et al.57 proposed an 8-color panel for assessing CLL MRD, including CD81FITC/CD38PE/CD20PerCP/CD43PECy7/CD5/APC/CD45APC-cy7/CD19V450/CD3 V500, which has shown good correlation with the 4-color ERIC approach and with ASO IgH real-time PCR (RQ-PCR), but it was tested in only a few patients and has not been validated by other centers. Although treatment protocols based on anti-CD20 monoclonal antibodies induce loss of CD20 expression, they do not affect the performance of flow cytometry MRD assays.58,59
Recommendations of the Brazilian Group of CLL for MDR evaluationTime points for MRD assessment: the recommendations are to perform MRD evaluation only in the context of translational research and clinical trials and usually to investigate MRD three months after completion of therapy regimens intended to eradicate leukemic clones.
Sample: either PB or bone marrow can be used for MRD assessment. However, there is a higher probability for BM to be MRD positive than PB, although the associated clinical significance is still unknown. Hemodilution and sample cellularity must be evaluated and taken into account. Sensitivity may be lower in hypocellular samples.
Acquisition: the number of acquired events in the flow cytometer must be at least 500,000 total events to achieve a sensitivity of 10−4 with at least 20 CD19+/CD5+ events needed to characterize the population being analyzed.
Panel of monoclonal antibodies: the recommendation of the Brazilian Group of CLL for MRD assessment is to use ERIC 4- or 6-color protocols based on their high reproducibility.
TreatmentWith a better understanding of CLL biology, there has been steady progress in treatment in recent years. Several new drugs have been approved with different mechanisms of action.
For both the indication of treatment and evaluation of therapy, the International Workshop on CLL (iwCLL) guidelines should be used.22,42
Although it cannot be pursued as a treatment goal due to a lack of data, the importance of MRD assessment is growing and is correlated with improved PFS and, potentially, OS.54,60
Indications for treatmentTo date, there is no evidence of a clinical benefit in treating CLL at diagnosis61 and treatment should be initiated only if there is a clear indication according to iwCLL criteria.22 It is important to clarify that all treatment indications should be cautiously judged as CLL-related. Treatment should be initiated only after other causes have been excluded, such as infectious diseases or other neoplastic diseases.
The treatment indications are as follows:
- 1.
Bone marrow failure manifested by the development of anemia and/or thrombocytopenia;
- 2.
Massive splenomegaly (at least 6cm below the left costal margin) that is progressive or symptomatic;
- 3.
Massive lymph node (at least 10cm in longest diameter) or a progressive or symptomatic lymph node;
- 4.
Progressive lymphocytosis with an increase of more than 50% within two months or a LDT of less than six months. LDT can be determined by linear regression extrapolation of absolute lymphocyte counts obtained at intervals of two weeks over an observation period of 2–3 months (this parameter should not be used if the initial lymphocyte count is less than 30×109/L). In addition, factors contributing to lymphocytosis or lymphadenopathy other than CLL (e.g., infections) should be excluded.
- 5.
Autoimmune disease (anemia and/or thrombocytopenia) with poor response to corticosteroids or other standard treatments.
- 6.
Constitutional symptoms, which are defined as any of the following: unintentional weight loss of 10% or more in the past six months, significant fatigue [i.e., Eastern Cooperative Oncology Group (ECOG) of 2 or worse; inability to work or perform usual activities], fever higher than 38.0°C for two or more weeks without evidence of infection, and night sweats for more than one month without evidence of infection.
To select the best treatment for each patient, it is important to evaluate not only disease stage and cytogenetic risk but also the patient's physical condition and comorbidities. A comorbidity scale can be used to classify patients as ‘Go-Go’, ‘Slow-Go’, and ‘No-Go’.42 One example of such a scale is the ‘cumulative illness rating scale’ (CIRS).62 In clinical trials, patients with a CIRS score ≤6 and a normal estimated glomerular filtration rate (creatinine clearance>70mL/min/1.73m2) are considered ‘fit’ for more intensive treatments.
ChlorambucilMonotherapy with alkylating agents, including chlorambucil, has been the treatment of choice for many years61, and this therapy can still be an option, particularly for elderly and unfit patients for whom current standard treatments are not an option. The advantages of chlorambucil include its low cost, low toxicity, and convenience of being an oral treatment. The main disadvantage is the very low, if any, rate of complete response and the risk of side effects with long-term use, such as myelodysplasia. Currently, the use of chlorambucil alone is avoided whenever a monoclonal antibody is available.42
Purine analogsPurine analogs are still one of the most important drugs in the treatment of fit patients, and fludarabine is the most studied drug. Response rates with fludarabine monotherapy range in different studies from 63 to 73% with approximately 7–40% of cases having a complete response, corresponding to a response that is superior to that achieved with chlorambucil.63 Long-term monitoring revealed a better OS for fludarabine,64 although a greater OS does not seem to be evident in older patients.65 Fludarabine monotherapy was also no less effective than more intensive regimens associated with alkylating agents.66,67
Combinations of purine analogs and alkylating agents have synergistic cytotoxicity in CLL because both chemotherapies have different mechanisms of action and different toxicity profiles. The combination of fludarabine with cyclophosphamide (FC) is the most studied therapy and yields better overall response (74–94%) and complete remission rates (23–38%) than other regimens in the pre-rituximab era without increasing the risk of infection despite a higher incidence of neutropenia.68–70 Other purine analogs have also been studied, but there was no significant benefit over fludarabine.71
Monoclonal antibodies: anti-CD20In recent decades, the addition of monoclonal antibodies has changed the treatment of all lymphoproliferative disorders, including CLL, after the introduction of rituximab in treatment protocols.
In CLL, rituximab is less active as a single agent than in other lymphoproliferative disorders, which is probably because of the low density of CD20 in the cell membrane. Therefore, higher doses of this monoclonal antibody are recommended for CLL.72,73 The combination of rituximab with fludarabine and cyclophosphamide (FCR) has a synergistic effect and efficacy that has been confirmed in several phase II studies and retrospective analyses. In the largest phase II study, FCR resulted in an overall response rate of 95%, complete remission rate of 72%, OS at six years of 77%, and medium PFS of 80 months.74 These results led the German group to conduct the CLL8 study that demonstrated the superiority of FCR compared with FC, with better response rates and better PFS rates as well as no increase in toxicity or infection risk. Subgroup analysis showed benefits for all cytogenetic risk factors, except for individuals with del17p.43 The same beneficial results associated with FC were also obtained with second-line treatment.75 These results made the FCR combination the treatment of choice for fit patients with CLL. Because CLL occurs at a high frequency in older patients, an FCR-Lite scheme has been designed in an attempt to reduce toxicity while maintaining efficacy. In this combination, fludarabine dosages are reduced to 20mg/m2, cyclophosphamide dosages are reduced to 150mg/m2 on Days 2–4 in cycle 1 and on Days 1–3 in cycles 2–5, and the dosages are increased for rituximab (375mg/m2 on day 1 of cycle 1 and 500mg/m2 on day 14 of the first cycle and on Days 1 and 14 in all subsequent cycles). After the six cycles were completed, rituximab was given as a maintenance therapy at 500mg/m2 once every three months until relapse.76
Ofatumumab is another monoclonal antibody that targets a specific epitope with increased affinity to CD20 and also with stronger complement-dependent cytotoxicity (CDC), as well as antibody-dependent cellular cytotoxicity (ADCC).77 This drug was approved in Europe and in the United States as a monotherapy for relapsed or refractory patients. It also has reasonable response rates in high-risk individuals, including those who are refractory to fludarabine and alemtuzumab or refractory to fludarabine and have bulky disease, with overall response rates of 58% and 47%, respectively.78 In the first-line treatment, the combination with chlorambucil in treatment-naïve unfit elderly patients yielded an excellent response rate of 82% with a 12% complete response rate.79
Obinutuzumab (GA101) is a humanized monoclonal antibody that showed impressive results in animal and pre-clinical studies, and it has been designed to have greater affinity for the type II CD20 epitope, greater ADCC, and lower CDC with higher direct cell death induction.80 The CLL11 study showed that the combination of obinutuzumab with chlorambucil yielded a response rate of 78.4% and a 20.7% complete response rate, with 19.5% MRD negativity in CLL patients not eligible for fludarabine-based treatment. It was far superior to chlorambucil alone and yielded better response rates than the combination of rituximab and chlorambucil.81 Obinutuzumab-related infusion reactions occur in nearly 65% of cases in the first cycle (21% grade 3 or 4), which leads to discontinuation in 7% of patients. The rate of infusion reactions drops to 3% in the second cycle and to less than 1% in subsequent cycles.
BendamustineMore recently, bendamustine, an alkylating agent with purine analog properties, was compared with chlorambucil in a multicenter, randomized trial and yielded a better response rate of 68% with a 31% complete response rate and PFS of 21.6 months. There was no difference in OS.82
Promising results were obtained from the combination of bendamustine with rituximab (BR) in relapsed CLL patients at a dose of 70mg/m2 of bendamustine on Days 1 and 2 and 375mg/m2 of rituximab on Day 1 of the first cycle and 500mg/m2 in the subsequent cycles, for a total of six cycles every 28 days.83 When used as first-line treatment, the BR scheme with a higher dose of 90mg/m2 of bendamustine yielded a 97% response rate and complete remission rate of 31%. A CLL10 study demonstrated comparable results to FCR in terms of response rates but with fewer complete remissions. This difference was not confirmed in the sub-analysis of patients older than 65 years or who had more comorbidities.84 Unfortunately, bendamustine is not available in Brazil yet and is not approved for use.
AlemtuzumabAlemtuzumab is a humanized monoclonal antibody against the CD52 antigen with proven activity in CLL. In patients with advanced-stage disease, alemtuzumab monotherapy yielded response rates of 30–50%, with a median PFS of 9–15 months after second-line treatment with fludarabine.85–87 Alemtuzumab also has proven effectiveness for both del11q and del17p.88 In a randomized study, alemtuzumab showed a higher response rate and better PFS in treatment-naïve patients than chlorambucil.89 The synergistic activity of alemtuzumab with fludarabine was effective and safe in a phase II study, with a response rate of 83% and complete remission rate with negative MRD of 53%.90 In a phase III study, alemtuzumab in combination with FC was more toxic than the FCR regimen.91 In a phase II study, a combination treatment of alemtuzumab with FCR demonstrated excellent response rates of 92%, with 70% of all individuals achieving complete remission and 57% of del17p individuals achieving complete remission. This scheme can be an alternative bridge therapy to achieve remission before transplantation in del17p patients.92 In a multicenter phase II study, the combination of 30mg alemtuzumab three times a week with 1.0g/m2 methylprednisolone (MP) for five consecutive days every 4 weeks in TP53-deleted CLL resulted in an overall response rate of 85% and a complete response rate of 36%. The risk of infection was age-related and seemed only marginally higher in younger patients than the infection risk associated with FCR.93
Alemtuzumab is no longer approved for use in Brazil for CLL and is marketed only for its indication in multiple sclerosis. However, alemtuzumab is available for patients with CLL through a compassionate-use program.
LenalidomideLenalidomide is a medication with immunomodulatory and antiangiogenic effects. It has reasonable response rates in relapsed patients94,95 and has some activity in del17p patients.96 Phase II trials demonstrated good synergism with rituximab.97 An association between rituximab and fludarabine is also feasible, although a phase I study yielded ominous results, with an excess of side effects, myelosuppression and tumor flares.98
Unfortunately, lenalidomide is not available in Brazil yet and is not approved for use.
Agents targeting B-cell receptor signalingA new therapeutic drug class has shown promising results when used in conjunction with targeted therapy in CLL. The most prominent agent in this class seems to be the Bruton's tyrosine kinase inhibitor ibrutinib. Phase I and phase II studies have shown a surprising response in relapsed or refractory patients, including high-risk groups (e.g., del17p patients or patients who relapsed within 24 months of previous treatment). PFS and OS at 26 months were 71% and 83%, respectively, and 57% and 70% in patients with del17p, respectively.99 The phase III RESONATE study included 391 relapsed or refractory patients, including 33% with del17p. Ibrutinib showed better PFS and OS results than ofatumumab. The excellent response rates were maintained in high-risk patients with del17p as well as in patients refractory to purine analogs. This medication is considered the best choice for second-line treatment in elderly patients and patients with comorbidities.100 The presence of lymphocytosis is common in ibrutinib patients, with peak lymphocytosis usually occurring four weeks after the beginning of treatment, but 80% of patients have had resolution or at least a 50% decrease in their lymphocyte count. This decline was faster in patients with unmutated IgHV.99 Ibrutinib treatment used as first-line treatment was tested in the RESONATE-2 study in elderly patients and was compared with chlorambucil, yielding excellent results. There was a 86% overall response rate, and OS was estimated at 24 months to be 98%.101 Combination therapy with rituximab102 and ofatumumab103 has been performed in relapsed patients with promising results, even in high-risk groups. The best approach for using these novel modalities is still a matter of debate. Ibrutinibe was recently approved in Brazil, only for relapsed/refractory CLL patients.
Another important medication is an inhibitor of the class I phosphatidylinositol 3-kinase (PI3K) p110 δ isoform called idelalisib. A phase I study in relapsed or refractory patients showed promising results, and a percentage of patients showed a partial response with lymphocytosis.104 Its combination with Rituximab was tested in treatment-naïve patients105 and in a relapse context.106 In a phase II study with idelalisib as a first-line treatment in elderly patients, combination treatment showed excellent results, with an overall response rate of 96.0% and a complete response rate of 14.1%, as well as PFS and OS rates at 36 months of 92.9% and 90%, respectively. The response was maintained in patients with del17p. The presence of diarrhea or colitis grade 3 or 4 can limit the use of this medication, mainly because the incidence of side effects was more common after multiple months of treatment.105 Idelalisib is not available in Brazil yet and is not approved for use.
Bcl-2 inhibitorsOther new therapeutic classes include Bcl-2 inhibitors. A phase I study with Venetoclax (ABT-199) showed excellent results, with an overall response rate of 79% and a 20% complete response rate, including 5% negativity for MRD and a PFS rate at 15 months of 66%. High-risk del17p patients had similar results, but with less sustained PFS.107 Venetoclax is not available in Brazil yet and is not approved for use.
Allogeneic hematopoietic stem cell transplantationAllogeneic HSCT is effective in CLL as shown by the 10-year complete remission rate of 69% and OS of 55% in a cohort of 49 consecutive patients with 20 years of follow up.108 However, the non-relapse mortality is still in the range of 20% in most series.109 An important finding is that prognostic factors that negatively influence the outcome of CLL under chemoimmunotherapy, such as unmutated IGHV gene, unfavorable genetic abnormalities, and purine analog refractoriness, do not adversely affect PFS or OS after HSCT.109 Currently, HSCT is still the only curative option for appropriate candidates (young, fit patients with an adequate donor). As there is no direct comparison between transplant and the novel agents (ibrutinib, idelalisib, or venotoclax), more data is needed to determine which patients should still be considered for HSCT, and which should be considered for prolonged treatment with one of these novel agents.
Treatment recommendations are shown schematically below. Patients classified as ‘Go-Go’ should receive combination therapy with FCR (preferable) or BR (especially for patients older than 65 years).
‘Slow-Go’ patients should receive chlorambucil in combination with an anti-CD20 antibody: rituximab, ofatumumab, or obinutuzumab (preferable). Alternative schemes include a fludarabine-containing regimen at a reduced dose, such as FCR-lite, or a combination of bendamustine and rituximab. The treatment goal is to control symptoms.
In patients with symptomatic disease and del17p or TP53 mutations, the therapy of choice is a kinase inhibitor, which is preferably ibrutinib. However, idelalisib in association with rituximab or the bcl-2 inhibitor venetoclax are acceptable alternatives if available. Alemtuzumab alone or in combination with MP also has good activity and can be used. Allogeneic HSCT should be considered in all patients in good clinical condition and if a matched donor is available.
The first treatment can be repeated if the time to relapse has extended past 24 months in patients who received chemoimmunotherapy.
In patients with refractory CLL or early relapse (<24 months) and in patients with del17p, the treatment should be changed.
The therapy of choice is ibrutinib. Alemtuzumab (alone or in combination with MP), idelalisib plus rituximab (for slow-go patients), and venetoclax (only in the presence of del17p or TP53 mutations) are acceptable alternatives, if available. Allogeneic HSCT should be considered the only curative option for all patients in good clinical condition if a matched donor is available.
Recommendations of the Brazilian Group of CLL for first and second-line treatment- 1)
First-line treatment:
- a)
‘Go-go’ patients:
- -
First choice: fludarabine, cyclophosphamide, and rituximab (FCR)
- •
Alternative options: bendamustine and rituximab (BR)
- •
- -
Del (17p) or TP53: ibrutinib and consider allogeneic HSCT
- •
Alternative options: idelalisib plus rituximab, venetoclax, alemtuzumab with or without high-dose methylprednisolone, rituximab with or without high-dose methylprednisolone
- •
- -
- b)
‘Slow-go’ patients:
- -
First choice: anti-CD20 antibody (obinutuzumab, ofatumumab, or rituximab) plus chlorambucil
- •
Alternative options: FCR-lite, BR.
- •
- -
Del (17p) or TP53: ibrutinib
- •
Alternative options: idelalisib plus rituximab, venetoclax, alemtuzumab high-dose methylprednisolone, rituximab with or without high-dose methylprednisolone
- •
- -
- a)
- 2)
Relapsed first-line treatment:
- a)
Progress after 24 months: repeat first-line treatment (add an anti-CD20 antibody if not used in the first-line treatment)
- b)
Progress within 24 months:
- -
‘Go-go’ patients: ibrutinib
- •
Alternative options: alemtuzumab with or without methylprednisolone, rituximab with or without high-dose methylprednisolone, allogeneic HCST, bendamustine plus rituximab
- •
- -
‘Slow-go’ patients: ibrutinib
- •
Alternative options: idelalisib plus rituximab, alemtuzumab with or without methylprednisolone, rituximab with or without high-dose methylprednisolone bendamustine plus rituximab, FCR-lite
- •
- -
- a)
The authors declare no conflicts of interest.