Successful transfusion of platelet refractory patients is a challenge. Many potential donors are needed to sustain human leukocyte antigen matched-platelet transfusion programs because of the different types of antigens and the constant needs of these patients. For a highly mixed population such as the Brazilian population, the pool size required to provide adequate platelet support is unknown.
MethodsA mathematical model was created to estimate the appropriate size of an unrelated donor pool to provide human leukocyte antigen-compatible platelet support for a Brazilian population. A group of 154 hematologic human leukocyte antigen-typed patients was used as the potential patient population and a database of 65,500 human leukocyte antigen-typed bone marrow registered donors was used as the donor population. Platelet compatibility was based on the grading system of Duquesnoy.
ResultsUsing the mathematical model, a pool containing 31,940, 1710 and 321 donors would be necessary to match more than 80% of the patients with at least five completely compatible (no cross-reactive group), partial compatible (one cross-reactive group) or less compatible (two cross-reactive group) donors, respectively.
ConclusionThe phenotypic diversity of the Brazilian population has probably made it more difficulty to find completely compatible donors. However, this heterogeneity seems to have facilitated finding donors when cross-reactive groups are accepted as proposed by the grading system of Duquesnoy. The results of this study may help to establish unrelated human leukocyte antigen-compatible platelet transfusions, a procedure not routinely performed in most Brazilian transfusion services.
Platelet alloimmunization is commonly seen in patients with hemato-oncological disorders requiring frequent red blood cell and platelet transfusions1 and may be associated with refractoriness to platelet transfusions (RPT). There may also be an association between platelet transfusion failure and patient survival, which increases the clinical importance of RPT.2
RPT is defined as inappropriately low platelet count increments following exposure to antigens after two or more (usually consecutive) transfusions and must be determined by objective data which determine platelet transfusion outcomes.3 This condition may be caused by immune and non-immune factors. More than 80% of RPT cases are related to non-immune causes. Thus, immune causes occur in less than 20% of the cases involving alloimmunization against human leukocyte antigens (HLA) and, to a lesser extent human platelet antigens (HPA), following exposure after transfusion, pregnancy, or transplantation. Among the immune causes, HLA antibodies are responsible for approximately 80–90% of RPT cases and HPA antibodies for approximately 10–20% of cases, associated or not with HLA antibodies.4
Providing an adequate post-transfusion platelet count increment to refractory patients is not an easy task; transfusion of HLA-matched platelets is one possibility.5 However, it is very difficult to find multiple HLA-compatible related donors for one individual.
The HLA system is highly polymorphic6 and the probability of finding identical matches may be around 10% of donations.7,8 When a full match cannot be found, different strategies are used to select partially HLA-matched donors. HLA class I specificities can be grouped into cross-reactive groups (CREG), mismatches with antigenic similarity that result in less allorecognition or immune activation.9 The grading system described by Duquesnoy et al. in the 1970s10 (Table 1) is defined according to the presence of HLA CREGs and is still widely used by transfusion services. Although in some cases, the selection of mismatched donors based on HLA CREGs may fail to produce adequate increments,11 this strategy can increase the number of potential donors in the same donor base.8
Description of the grading system of Duquesnoy.
| Grade | Description | R/D | HLA typing | |||
|---|---|---|---|---|---|---|
| R | A1 | A2 | B7 | B8 | ||
| A | HLA identical – all 4 antigens | D | A1 | A2 | B7 | B8 |
| BU | Only 3 antigens detected – all identical | D | A1 | – | B7 | B8 |
| B2U | Only 2 antigens detected – both identical | D | A1 | – | B8 | – |
| BX | 4 antigens detected – 3 antigens identical and 1 cross-reactive | D | A1 | A24 | B7 | B8 |
| BUX | 3 antigens detected – 2 identical and 1 cross-reactive | D | A1 | A24 | – | B8 |
| B2X | 4 antigens detected – 2 antigens identical and 2 cross-reactive | D | A1 | A24 | B7 | B64 |
| C | 1 antigen mismatch, out-of-CREG | D | A1 | A32 | B7 | B8 |
| D | All other ≥2 antigen mismatches | D | A1 | A32 | B7 | B64 |
R: recipient; D: donor; HLA: human leukocyte antigen; –: undetected antigens.
Pool size calculations can be an essential component for the rational planning of platelet support programs.12 It is estimated that to provide at least five completely compatible donors for more than 80% of patients, 500, 1000, and 1500 donors would be needed for the Japanese, European Caucasoid and North American Caucasoid populations, respectively.13 However, for a highly mixed population such as in Brazil, which is comprised of European, African and Amerindian roots,14 the pool size required to provide these patients with adequate platelet support is unknown.
The unrelated donor pool size that might be necessary if a center wants to provide patients with unrelated HLA-compatible platelets was estimated using a random sample from the Brazilian population. A mathematical model was created for compatibility analysis and its application was illustrated in a population of 154 cancer patients. The findings of this study may help to establish the transfusion of unrelated HLA-compatible platelets, which currently is not a routine procedure in many Brazilian centers.
MethodsStudy database, design and settingA group of 154 HLA-typed patients who were submitted to bone marrow transplantation or who were candidates for this procedure at Hospital Israelita Albert Einstein (São Paulo, Brazil) between January 2006 and December 2009 were included in this retrospective study to illustrate a possible patient population.
A database of 65,500 HLA-typed bone marrow donors, registered in the LIG Laboratório de Imunogenética Ltda, São Paulo, Brazil was used in this study as the potential donor population. This database includes samples from the southeastern (mainly), southern and northeastern regions of Brazil and represents a section of the National Registry of Bone Marrow Donors. According to a Brazilian demographic census, these regions are related to 80% of the population15 and may represent a good picture of the HLA phenotype diversity of the Brazilian population. This study was approved by an Ethics Committee and the Local Review Committee.
Measures and statistical analysisPatients and donorsHLA typing was performed by the polymerase chain reaction sequence specific oligonucleotide probe (PCR-SSOP) method for loci A and B.
In order to search the donors to match each of the 154 patients automatically, a mathematical model was generated using a Visual Basic computer program.16 Platelet HLA compatibility was based on the grading system of Duquesnoy and HLA cross-reactive antigens were used as described in Table 1.17,18
Donors for each patient were grouped according to the compatibility found defined as completely compatible (CC) for matches A, B1U, B2U (no CREG present), partially compatible (PC) for B1X, B2UX matches (only one CREG present) and less compatible (LC) for B2X matches (two CREGs present). The results obtained of two examples from the mathematical model validation process are described in Table 2.
Examples of the compatibility using the grading system of Duquesnoy.
| Recipient | Duquesnoy classification | Number of donors | Compatibility type | n |
|---|---|---|---|---|
| 1 | A | 1 | CC | 10 |
| B1U | 0 | |||
| B2U | 9 | |||
| B1X | 52 | PC | 93 | |
| B2UX | 41 | |||
| B2X | 571 | LC | 571 | |
| 2 | A | 2 | CC | 159 |
| B1U | 51 | |||
| B2U | 106 | |||
| B1X | 230 | PC | 848 | |
| B2UX | 618 | |||
| B2X | 1565 | LC | 1565 | |
CC: complete compatible; PC: partial compatible; LC: less compatible.
An estimation of the required number of donors for each patient was calculated using binomial distribution with parameters given by the proportion of compatibility observed in the donor population. The curves to estimate the donor pool size were built according to the percentage of patients with at least one and five compatible donors for each simulated pool size. Details of the mathematical model are available upon request.
The projection model was applied in order to define how large the donor pool should be to provide at least five CC, five PC or five LC donors for 80% of the patient population, which was considered an acceptable number of donors for platelet support during the thrombocytopenic period.
Validation of the mathematical modelThe mathematical model was validated by randomly using 10% (15/154) of the patient group. Two HLA experts selected compatible donors based on the CREG definition and manually grouped them based on the grading system of Duquesnoy using the filter tool of the Excel program. The same compatible donors were obtained both by manual selection and by the mathematical model. During this validation period there were no errors due to failures of the model, therefore, the automation tool provided reliable histocompatibility analyses.
ResultsProbability for finding matched donorsThe compatibility program revealed that of the 154 patients, 141 (91.6%) had at least five CC in the database of 65,500 registered donors, and all patients (100%) had at least five PC and LC donors.
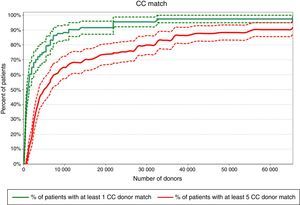
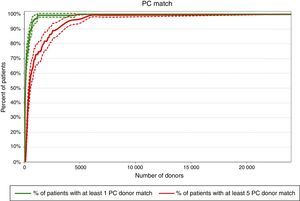
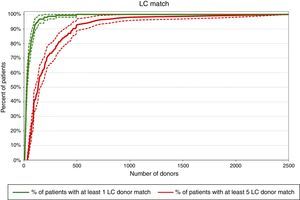
Required pool size to match 80% of the patient population with at least one donorFigures 1–3 show that according to this projection model it is necessary to have a pool containing 6502, 341 and 65 donors, respectively to match a minimum of 80% of the patients with at least one CC, PC or LC donor. More than 65,500 donors would be necessary to find at least one CC donor for all patients (100% success rate).
Pools of 31,940, 1710 and 321 donors would be necessary to provide at least five CC, PC or LC donors to 80% of the patients, respectively according to the projection model (Figures 1–3). Pools of 23,393 and 2500 donors would be enough to match 100% of the patients with five PC and LC donors, respectively.
DiscussionThe present projection model shows that pools of 31,940, 1710 and 321 donors would be necessary to match 80% of the patients with at least five CC, PC or LC donors, respectively. This calculation, based on a different mathematical model, has already been performed for the Japanese, European Caucasoid and North American Caucasoid populations in which 500, 1000, and 1500 donor candidates would be needed to find at least five LC donors for more than 80% of each of these populations. On the other hand, to find at least five CC, 5000, 18,000 and 25,000 preselected donor candidates would be necessary for these populations, respectively.13 In another study, the authors concluded that 1500 platelet donors would be required to supply 75% of the patients with eight LC donors in the North American Caucasoid population. This calculation would meet the transfusion needs of community donor platelet apheresis programs in a reference center (Seattle) of the United States.19
Brazil's ethnic and genetic heterogeneity, which is related to the allelic variants present in the first populations that inhabited the country,20 combined with the existence of many HLA polymorphisms, has most likely made it more difficult to find at least one CC, even when a database of 65,500 individuals is used. However, this heterogeneity seems to have acted as a facilitator when cross-reactive antigens are accepted, as in the grading system of Duquesnoy.
BX or B2X mismatched products have already been reported as an acceptable match for platelet transfusions when the recipients’ lymphocytotoxic antibodies have low reactivity,21 even though this type of blood product can increase the chances of alloimmunization and make future transfusions difficult. This study shows that, for this level of compatibility, the pool may be feasible and should have 321–1710 donors. However, to provide five matching CC donors, a larger number of donors would be needed (31,940). These data support the strategy of including any single cross-reactive antigen while selecting donors, particularly if the patient to be transfused presents low titers of antibodies and consequently a low probability of alloimmunization.
Successful transfusion of patients with platelet refractory thrombocytopenia is extremely important. However, many potential donors are needed to sustain HLA-matched platelet transfusion programs because of the considerable variety of HLA types and the constant needs of these patients. The question of the required donor pool size should also consider feasibility and costs.12 The latter is one of the reasons why there are no well-established unrelated HLA-matched platelet transfusion programs in most Brazilian services. Pool size calculations may provide essential data for rational planning of platelet transfusion support programs and guide institutions that aim to build a platelet donor registry.
The use of HLA-matched platelets is not the only approach used to manage alloimmune RPT. Crossmatching and support with antigen negative platelet units allow rapid selection of donors, mainly in patients with uncommon HLA types for whom it might be virtually impossible to find HLA-compatible donors.22–24 Recently, the use of the HLAMatchmaker algorithm has been reported as an emerging concept for the management of refractory patients.25,26 The combination of matching compatible antigens and the application of mismatch acceptability determined by serum screening for HLA antibodies has offered an effective approach to an HLA-based platelet transfusion support policy for refractory patients.27,28 The lack of antibody specificity is a major limitation in this study as it does not account for the relative frequencies of certain antibodies in the population.
Although the frequency of immune refractoriness has declined during the past decade due to the application of universal leukoreduction of platelet preparations,2,29 RPT is a complex process and poses a great challenge in the treatment of thrombocytopenic patients. However, universal blood leukoreduction is not frequent in the transfusion practices of Brazil and thus RPT is still a difficult nationwide problem. Knowing how large the donor pool has to be, may help and stimulate different centers in Brazil to build unrelated platelet donor panels.
In conclusion, according to the projection model, 31,940 and 321 donors would be necessary to provide at least five CC or LC donors, respectively to 80% of the patients in the Brazilian population. Furthermore, 23,393 and 2500 donors would be enough to match 100% of the patients with five PC and LC donors, respectively. On the other hand, the CC pool size to match 100% of patients is not possible to calculate possibly because of the great racial miscegenation of Brazilians.
Conflicts of interestThe authors declare no conflicts of interest.