The treatment and evolution of B-cell non-Hodgkin lymphoma (B-NHL) has undergone important changes in the last years with the emergence of targeted therapies, such as monoclonal antibodies, small molecules, antibody-drug conjugates, and bispecific antibodies. Nevertheless, a significant portion of patients remains refractory or relapsed (R/R) to the new therapeutic modalities, representing thus an unmet medical need. The use of CAR-T cells for the treatment of B-NHL patients has shown to be a promising therapy with impressive results in patients with R/R disease. The expectations are as high as the imminent approval of CAR-T cell therapy in Brazil, which it is expected to impact the prognosis of R/R B-NHL. The aim of this manuscript is to offer a consensus of specialists in the field of onco-hematology and cellular therapy, working in Brazil and United States, in order to discuss and offer recommendations in the present setting of the use of CAR-T cells for patients with B-NHL.
Care of B-cell non-Hodgkin lymphomas has undergone substantial changes over the last decade with introduction of targeted therapies such as monoclonal antibodies, small molecules, antibody-drug conjugates, and bispecific antibodies.1 Despite recent advances, many patients still succumb to the disease with most deaths directly related to refractoriness to therapy,2 emphasizing the unmet need for new strategies to address refractory and multiply relapsed (R/R) presentations.
From the histopathological point of view, diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for 25–40% of cases.3 Although most patients with DLBCL treated in the immunochemotherapy era respond to treatment, 20–40% of patients will either fail to achieve remission or will relapse.4 Patients with primary refractory DLBCL (PRD) have a particularly dismal prognosis. In the SCHOLAR-1 trial, an international multi-cohort retrospective study, patients with PRD, defined as progressive or stable disease (PD or SD) as best response to chemotherapy and/or relapse up to 12 months after autologous hematopoietic stem cell transplantation (ASCT), had poor outcomes with standard salvage therapies with overall response (OR) and complete response (CR) rates of 26% and 7%, respectively, and median overall survival (OS) of 6.3 months.2
Relapsed/refractory mantle cell lymphoma (MCL) also represents a great challenge.5 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been considered a theoretically curative option reserved primarily for young patients who achieve a second response with rescue regimens.2 Nevertheless, its role has decreased significantly over the last few years considering the severe toxicity (10–24% treatment related mortality), high relapse rates (3-year PFS of 30–40%),6–8 and efficacy of newer agents. However, even Bruton tyrosine kinase (BTK) inhibitors, the most effective class in MCL in monotherapy, still provide limited disease control emphasizing the need for novel therapies.
Low-grade B-cell NHL (LG-NHL) are a group of heterogeneous mature B-cell lymphomas with indolent behavior that often affect older patients. Relapses are common and the prognosis is poor in patients with early relapses.
Chimeric antigen receptor T-cells (CAR-T) are T lymphocytes genetically modified to recognize a specific antigen, independently of human leukocyte antigen (HLA) with targets including, for example, CD19, an antigen expressed on most B-lymphocytes. In 2017, the first commercial CAR-T cell product was approved by the US Food and Drug Administration (FDA) for the treatment of R/R DLBCL after at least two treatment lines - axicabtagene ciloleucel (axi-cel).9 Two additional products – tisagenlecleucel (tisa-cel) and lisocabtagene maraleucel (liso-cel) – have been approved for the same indication with CR and 12-month progression-free survival (PFS) rates ranging between 40–64% and 44–49%, respectively (Table 1).10–13 Other CAR-T cell products have subsequently been approved for the treatment of other B-cell lymphoma subtypes – brexucabtagene autoleucel (brexu-cel) for R/R MCL and axi-cel for follicular lymphoma (FL) after two or more therapy lines14–17 – with evidence of activity in other LG-NHL and better tolerability than in more aggressive histologies.17
Studies that led to approval of CAR-T cell therapy in B-cell non-Hodgkin lymphoma in the US.
CR: complete remission; CrCl: creatinine clearance; CRS: cytokine release syndrome; EF: ejection fraction; DLBCL: diffuse large B-cell lymphoma; MZL: marginal zone lymphoma; PMBCL: primary mediastinal large B-cell lymphoma; tFL: transformed follicular lymphoma; HGBCL: high-grade C-cell lymphoma; MCL: mantle cell lymphoma; ANC: neutrophil count; ALC; lymphocyte count; OS: overall survival; PFS: progression free survival; CR: complete response; RP: partial response; ECOG: Eastern Cooperative Oncology Group status performance.
There are high expectations that the imminent introduction of CAR-T therapy will help improve Brazilian lymphoma outcomes. The significant complexity of cellular therapy, however, represents a critical challenge for its proper implementation and for achieving these goals. This consensus is based on the expert opinion of lymphoma specialists, working in Brazil and in the United States, who have reviewed the current data that support the use of CAR-T cell therapy in B-NHL and propose a preferential approach to the different stages of anti-CD19 CAR-T cell implementation in Brazil.
DLBCLAnti-CD19 CAR-T cells have shown remarkable efficacy in R/R DLBCL. The three FDA-approved anti-CD19 CAR-T cell products have different features in terms of design and manufacturing process despite sharing the same antigen binding domain (murine-derived FMC63 antibody). The costimulatory domain (CD28 for axi-cel and 4-1BB for tisa-cel and liso-cel) is the most important difference in the CAR-T cell design with direct impact on the incidence and severity of CAR-T cell related toxicities – cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) – as well as on CAR-T cell persistence.
Cross-trial comparison between CAR-T cell studies in DLBCL has proven to be challenging not only due to these distinctions but also due to differences in trial design and patient selection. For instance, when comparing the manufacturing process of the different CAR-T cell products, the initial lymphocytes obtained through plasmapheresis can be cryopreserved, which adds logistical flexibility but might have an impact on manufacturing reliability and time. Manufacturing failures were rare in the trials ZUMA-1 (axi-cel pivotal trial) and TRANSCEND (liso-cel pivotal trial), but occurred in about 7% of the patients enrolled in the JULIET trial (tisa-cel pivotal trial). Deaths from disease progression, during T-cell manufacture, occurred in 10% of patients in JULIET and TRANSCEND trials, showing the aggressiveness of the disease and maybe higher risk patient population compared to the ZUMA-1 trial (Table 1).2,10,18,19
Among the patients who received anti-CD19 CAR-T cells in the different clinical trials, the OR and CR rates ranged from 52–83% and 40–58%, respectively. Durable remissions were seen in a subset of patients, particularly those achieving CR, with a plateau observed after 18 months in 35–40% of patients, suggesting that some patients might have been cured with this approach. The three FDA- approved CAR-T cell products demonstrated a remarkable gain in OS when compared to the outcomes of chemoimmumotherapy from the SCHOLAR-1 trial (Table 1).2,10,18,19
Even considering that the occurrence of CRS and ICANS is clearly related to the cell product construct and that there were higher incidence and severity of CRS and ICANS (and, consequently, the use of tocilizumab and corticosteroids) in the axi-cel trials (Table 1),2,10,17,19 it is difficult to compare toxicity between products and across different studies, once grading systems and toxicity management varied significantly. Current real world experiences have allowed the comparison of toxicity profiles between products from the union of hematology societies to create uniform grading criteria and toxicity management.
MCLBrexucabtagene autoleucel was the first engineered cell therapy product approved by the FDA for the treatment of MCL based on the results of ZUMA-2,19 a multicenter phase 2 open-label trial evaluating the anti-CD19 CAR-T therapy in R/R MCL patients relapsing after up to five previous therapies including BTK inhibitors. Sixty-eight of the 74 enrolled patients (92%) received the infusion of CAR-T cells, most of them presented high-risk features (TP53 mutation, Ki-67 > 30%, blastoid/pleomorphic histology in 17%, 82%, and 31% of the cases, respectively). OR in all patients was 85% with 59% CR and estimated PFS and OS at 1 year of 61% and 83%, respectively. The main cause of death was disease progression (21%), followed by infectious complications in two patients (3%). All patients experienced at least one adverse event, the most frequent being hematological toxicities. CRS and ICANS occurred in 91% and 63% of patients, respectively, although no deaths related to CRS or neurologic events were observed.16 Preliminary data from the TRANSCEND-NHL-001 study, a pivotal clinical trial evaluating liso-cel in the same setting, has shown promising results with an acceptable toxicity profile and very high response rates.20
CAR-T cell therapy will likely transform the therapeutic landscape and the role of transplant in R/R MCL. Nevertheless, there are still issues to be considered: CAR-T treatment is feasible and effective in patients with active disease, but the follow-up is short in comparison with allogeneic hematopoietic stem cell transplant (allo-HSCT). The allo-HSCT relies on the availability of a donor, not necessary in CAR-T cell therapy, and the rate of failure in the manufacture of brexu-cel was only 4%.21,22 Even the role of auto-HSCT is now being questioned in MCL. Recent reviews with real world data including 3,455 patients have demonstrated no impact of auto-HSCT on OS.23 Finally, the relevant economic impact and accessibility to CAR-T cells, both probably limited in Brazil at first, must be considered. The integration of CAR-T cell therapy into the MCL treatment algorithm is still far from being fully established. Long-term follow-up data and future studies will be critical to define the best setting for CAR-T cell therapy in MCL.
LG-NHLData on the use of anti-CD19 CAR-T cell therapy in FL has so far been presented only in abstracts, but it was impressive enough to lead to an FDA accelerated approval. In the preliminary analysis of 146 patients after a median follow-up of 17.5 months, including 84 patients with FL, OR was 94% with 80% CR with no impact of POD24, refractory status, or number of previous treatment lines in response. The median duration of response had not been reached by the time of data cutoff with 12-month estimated PFS of 74% and OS 93%. Grade 3 or higher CRS was observed in 6% of patients, and in 15% of patients with FL, with two deaths (multi-organ failure and aortic dissection not related to axi-cel), clearly lower than what was observed with axi-cel in high-grade B-cell NHL.17
Additional reports have suggested activity of cellular therapy in LG-NHL. In a recent review of 21 patients treated with CAR-T cell therapy, 8 with FL, CR rate was 88% with all those who achieved CR remaining in remission after a median follow-up of 24 months. Both CRS and ICANS occurred in 50% of patients, with no severe adverse events.14 Excellent responses have also been seen in chronic lymphocytic leukemia (CLL) with OR rate of 74%, including 21% CR, highlighting the potential of anti-CD19 CAR-T cells in this setting.24–27
Patient selectionThe most recent approvals for the treatment of FL and MCL have not provided sufficient time yet for real world experience. On the other hand, commercial CAR-T cell products have been commercially available since 2017 and extensive real world data have confirmed their effectiveness with response and survival rates very similar to the original clinical trials.5,27 FDA-approved package inserts, however, do not define any specific eligibility criteria beyond the number of previous treatment lines, although real-world experience has demonstrated that proper patient selection is a crucial step for successful cellular therapy outcomes.
Two separate issues play a critical role in patient selection – organ function and predictive markers of response. Retrospective data of patients treated with commercial products have demonstrated a trend of lower response rates and duration of response for those who did not meet the original eligibility criteria of the clinical trials that led to approval of these products.18 While impressive responses have been seen, even in highly refractory and bulky presentations, poorly controlled lymphoma requiring bridging, high markers of cell turnover or inflammation (such as lactate dehydrogenase-DHL, ferritin, and C-reactive protein), large tumor burden with extensive extranodal involvement or large metabolic tumor volume negatively influence the outcomes of CAR-T cell treatment in B-LNH.19,28,29
While we still try to establish the best sequencing of cellular therapy treatment and the best recently FDA-approved salvage agents, such as the anti-CD19 antibody tafasitamab or the antibody-drug conjugates, polatuzumab and loncastuximab, the progressively expanding option list at the very least allows for more careful selection of the best candidates for CAR-T cell therapy.
This is particularly challenging in the Brazilian healthcare system where many of these agents are still not available and the access to CAR-T cell therapy will, at least at first, be very limited. It will be fundamental that providers consider these features when selecting the patients to be treated with cellular therapy in the R/R B-LNH setting.
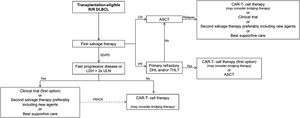
Figure 1 proposes an approach for patient selection for the treatment with anti-CD19 CAR-T cell therapy in the current Brazilian setting.30–32 AHSCT remains the standard of care as second-line therapy in the management of R/R DLBCL. Every patient considered eligible for AHSCT should undergo a prior salvage chemoimmunotherapy scheme outside of a clinical trial. Those with CR, particularly those with metabolic CR by PET/CT, should undergo consolidation AHSCT. Those patients with PR could still be considered for AHSCT, but those with disease characteristics associated with high chemorefractoriness, such as high-grade double-hit or triple-hit B-NHL or primarily refractory lymphoma, should be considered for CAR-T cell therapy. Likewise, patients with SD or PD, after salvage therapy, should be preferably considered for cellular therapy.
Treatment algorithm recommended for R/R DLBCL management.30
Algorithm for management of relapse/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) adapted from Alencar AJ et al.31 Treatment response was based on the LYRIC criteria (Cheson BD et al.32). Allogeneic hematopoietic stem cell transplantation may be considered instead of autologous hematopoietic stem cell transplantation (auto-HSCT) in selected cases of mobilization failure.
CAR, chimeric antigen receptor; CR, complete response; DHL, double-hit lymphoma; LDH, lactate dehydrogenase; PR, partial response; SD/PD, stable/progressive disease; THL, triple-hit lymphoma; ULN, upper limit of normal.
In view of failures after CAR-T cell treatment, more frequently observed in patients with fast progressive disease, alternative options, such as second salvage with novel agents or some bridging therapy (BT) in an attempt to achieve better disease control, should be considered prior to CAR-T cell therapy. This proposed approach aims to select the best candidates who may benefit the most from CAR-T cell therapy at least, in an initial phase in Brazil, when product availability will be extremely limited and successful outcomes will be fundamental to its consistent implementation.
Bridging strategies prior to CAR-T cell treatment for B-NHLMany patients who are candidates to CAR-T cell therapy have symptomatic disease that can be fatal if left untreated during the cell-manufacturing period. Bridging therapy (BT) – therapy administered after apheresis and before CAR-T cell infusion – may include corticosteroids, chemotherapy, targeted therapy, or radiation therapy (RT). According to the “Best practices recommendation of the European Society for Blood and Marrow Transplantation”, the goal of BT is to avoid clinically significant disease progression leading to impaired organ function or any other complications that might prevent the patient proceeding with lymphodepletion and receiving the CAR T-cells.
BT should ideally not induce major complications, such as infections, bleeding, or any organ dysfunction that might interfere with the planned lymphodepleting therapy and CAR T-cell infusion. BT can be omitted if there is stable/low burden disease and/or turn-around time for CAR T cell manufacture is expected to be short. Immunosuppressive drugs with a longer half-life, such as alemtuzumab, daratumumab, checkpoint inhibitors, or brentuximab vedotin, may interfere with the expansion or persistence of the infused CAR T-cells and should be avoided. When choosing BT for lymphoma, patient factors to be considered include prior response to chemo-immunotherapy, overall tumor burden, distribution and sites of tumor involvement. While parenteral agents, such as rituximab, gemcitabine, oxaliplatin, bendamustine or even oral chemotherapy as etoposide, cyclophosfamide and novel targeted agents as lenalidomide and ibrutinib, may be considered; high dose of corticosteroids for four days, repeated as needed, or RT for symptomatic or large masses, tend to be favored as they avoid additional unnecessary myelosuppression in patients that are highly chemorefractory.33
It is still unclear if BT affects the outcome of CAR-T cell therapy.34 The US Lymphoma CAR-T Consortium retrospectively evaluated the influence of BT in 298 R/R DLBCL cases intended to be treated with axi-cel at 17 academic institutions. BT, which was not permitted in the ZUMA-1 trial and led to axi-cel approval, was used in 53% of the patients. Of these, 54% received chemotherapy with or without other therapies, 23% used corticosteroids, 12% received RT with or without corticosteroids, and 10% underwent targeted therapies, such as lenalidomide or ibrutinib alone. BT was associated with worse OS. Patients who received BT were more likely to have poor prognostic features at the time of apheresis, such as ECOG performance status of 2–3, international prognostic index (IPI) ≥ 3, bulky disease, or elevated LDH.11
Preclinical studies have suggested potential synergy between RT and CAR-T cell therapy. Low doses of RT appear to sensitize negative tumor cells to antigen for CAR-T mediated apoptosis by making tumor cells susceptible to cell death mediated by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).35 RT also enhances cytotoxic T-cell migration to irradiated areas, reverses T-cell exhaustion, and diversifies the T-cell receptor repertoire of tumor infiltrating lymphocytes (TIL).36 In addition, complementary immunomodulatory activity, through induction of increased major histocompatibility complex (MHC)-1 expression and release of antigen by irradiated cells, may enhance tumor-specific immunity in irradiated and distant sites.31,37
Retrospective studies have evaluated the role of RT as BT for CAR-T cell therapy. In a retrospective review of 12 patients intended to receive RT as bridging prior to axi-cel therapy, 10 received RT after apheresis and 7 received concurrent systemic treatment. Eleven patients successfully received axi-cel infusion. There was a trend toward the decrease of PFS in 1-year among those who received any type of BT (29% compared to 44% for those who did not receive BT (p = 0.06).38 In a second review of 148 patients, 124 (84%) were successfully treated with CAR-T cell therapy, half required BT. Single modality RT bridging (n = 11, 65%) was associated with higher OR and CR rates, compared to systemic therapy alone (n = 6, 35%) (OR of 100% x 67%, p = 0.03 and CR of 82% x 38%, p = 0.01) and compared to the non-bridging cohort (n = 62) (OR of 100% x 82%, p = 0.13 and CR of 82% x 48%, p = 0.04).34,39
In a third retrospective study of 46 patients with R/R DLBCL treated with commercial anti-CD19 CAR T-cells – axi-cel (n = 21), tisa-cel (n = 25), BT was divided in two groups: high intensity BT (HI), including chemotherapy +/- immunotherapy, and low intensity BT (LI), including the monoclonal antibodies rituximab and brentuximab, lenalidomide and dexamethasone. Thirty patients (65%) received HI and 16 (35%) received LI or no bridging therapy. Patients who received HI had worse prognostic factors. Only 2 of the 46 patients could not receive CAR-T cell infusion as planned. There was no difference in response to cellular therapy between HI and LI groups with 57% progressing during BT. CRS and ICANS rates were higher in the HI group.40
New agents have also been tested as BT. In a retrospective study of 26 German centers, polatuzumab vedotin with bendamustine and rituximab (Pola-BR) was used as BT in 41 patients. In this cohort, 51.2% of patients were successfully bridged to intended CAR T-cell therapy resulting in a 6-month OS of 77.9% from the beginning of BT. Polatuzumab vedotin alone demonstrated ORR of 40% and may be considered a BT option as well.41
Finally, the T-cell modulation effect of ibrutinib may impact CAR-T cell outcomes. In human xenograft models of resistant acute lymphoblastic leukemia (ALL) and CLL, ibrutinib improved CAR T-cell engraftment and tumor clearance. In a phase 1/2 trial of patients with R/R CLL, the introduction of ibrutinib in CAR-T cell therapy decreased incidence of severe CRS and increased the response rate (88% versus 56%, p = 0.06), making ibrutinib an interesting BT strategy in CLL and MCL.42 Ongoing studies are evaluating the clinical significance of these findings. It is also important to note that this effect may not be universal to all agents in the class once newer molecules have more selective BTK inhibition.
CAR-T cell therapy incorporation by Brazilian public and private health systemsSeveral challenges are expected to the successful implementation of this modality of cellular therapy in emerging countries where public and private health systems coexist, such as Brazil.
The four commercially available anti-CD19 CAR-T cell products in the United States are priced between US$ 373,000 and US$ 475,000 for manufacturing alone.43,44 Separate ancillary, provider, and hospital charges can easily double the cost associated with these interventions. Reimbursement modalities based on outcomes have been implemented in multiple countries including the United States44 and Europe45 and seem to be the best approach to minimize this financial impact. Still, cost-effectiveness studies have demonstrated that CAR-T cell therapy can be cost-effective when compared to multiple consecutive treatment lines, which are also associated with high costs but inferior outcomes when compared to CAR-T cells.44,46
While alternatives to commercial products, such as research protocols with locally manufactured products, may improve the access in a public healthcare system, such initiatives still demand significant financial and intellectual investment.
Structure and quality represent additional expected barriers. Accreditation programs, such as FACT, JACIE and AABB, recommended for CAR-T centers, as well as extensive multi-disciplinary training and infrastructure adjustments for cell processing push up implementation costs.33 Therefore, it has been a natural trend in Europe and the United States to have cellular therapy centers developed in sites with extensive allo-HSCT expertise. Logistics will be another particularly important barrier to the implementation of CAR-T cell therapy in Brazil as patient referral to specialized centers will be challenging in a country with continental dimensions and limited resources.
Despite all challenges, CAR-T cell therapy is expected to be available soon for lymphoma patients in Brazil. Patient registry and post marketing studies must be considered as an important tool for surveillance of outcomes and progressive refinement of the processes involved.33,46
ConclusionAnti-CD19 CAR-T cell therapy has revolutionized the treatment landscape in relapsed or refractory B-cell NHL and it is a safe and effective option in this poor prognosis scenario. Adequate patient selection is crucial to assure that this expensive therapy with limited availability will be offered for patients who are most likely to benefit from it. Particularly in DLBC, where more data is available and alternative options are still scarce, an algorithm for management of relapse/refractory disease incorporating CD19 CAR-T cells is recommended by this Consensus. However, it may evolve over time, as new effective salvage non-chemotherapy agents are coming and trials evaluating the role of AHSCT are ongoing. Likewise, more data is needed to integrate anti-CD19 CAR-T cells into other B-NHL treatment algorithms.