Chimeric antigen receptor T (CAR-T) cell therapy is a novel therapeutic modality for acute lymphoblastic leukemia (ALL) with robust outcomes in patients with refractory or relapsed disease. At the same time, CAR-T cell therapy is associated with unique and potentially fatal toxicities, such as cytokine release syndrome (CRS) and neurological toxicities (ICANS). This manuscript aims to provide a consensus of specialists in the fields of Hematology Oncology and Cellular Therapy to make recommendations on the current scenario of the use of CAR-T cells in patients with ALL.
Acute lymphoblastic leukemia (ALL) is the most prevalent cancer among children. Intensive chemotherapy protocols offer cure to most of them.1 However, patients who develop refractory disease or early medullary relapse have a grim prognosis.2 According to the Brazilian National Cancer Institute, 524 children between 0 and 19 years of age died due to ALL in the country in 2019, whereas the number of adult deaths in the same year was 1, 710 (supplementary material).3
Despite important advances in clinical support to these patient population, the mortality rate of patients with ALL has not significantly decreased in our country in the last 15 years.3 The toxicities related to chemotherapy undermine any potential benefit with an increase in the treatment intensity. In this context, immunotherapy against different antigens on the leukemic cell surface has revolutionized the ALL treatment with significantly decrease in the toxicities compared to conventional chemotherapy.4
Currently, three immunotherapy modalities have been approved for clinical use in B-cell lineage ALL (B-ALL) in North America and Europe: an anti-CD22 antibody linked to calicheamicin (inotuzumab ozogamicin), a bispecific anti-CD3 and anti-CD19 antibody (blinatumomab) and an anti-CD19 chimeric antigen receptor T (CAR-T) cell product. The effect of inotuzumab depends on the calicheamicin, a chemotherapeutic agent with the capacity of inducing apoptosis of the target cells. The CD22 antigen has a slightly more restricted expression than CD19 in B-ALL, which may limit its use in some cases. Blinatumomab exerts an antileukemic effect upon engaging the patient's normal T cells against leukemic CD19+ cells. Its use as a monotherapy or, more recently, in association with chemotherapy (in pediatric patients in first early relapse) is associated with complete remission (CR) and minimal residual disease (MRD) negative rates that are superior to those of chemotherapy alone.5,6 For patients who have reached CR with negative MRD, consolidation with an allogeneic hematopoietic stem cell transplant (HSCT) is recommended. Each blinatumomab cycle lasts 28 days of continuous infusion, making the logistics of administration quite complex. In this context, the most recent and promising technology involves the genetic modification of autologous T cells, the CAR-T cells, to recognize and destroy cells of leukemic origin. This therapy results in high rates of MRD-negative CR.7 Considering the imminent approval of some commercial anti-CD19 CAR-T cell products by the Agência Nacional de Vigilância Sanitária (ANVISA), the Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (ABHH) invited a panel of specialists in hematological neoplasms, cellular therapy and HSCT to elaborate recommendations for this new treatment modality for B-ALL patients in Brazil.
In this manuscript, we describe practical recommendations with the objective of guide the selection of patients who will undergo anti-CD19 CAR-T cell therapy, as well as the evanulation and management pre- and post-infusion in the Brazilian scenario.
IndicationsThe use of CAR-T cells for the treatment of patients with B-ALL results in high response rates in patients with relapsed or refractory (R/R) disease.7 The first, and to date the only, product approved for commercialization by the American and European medication control agencies, namely, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of patients with B-ALL is tisagenlecleucel (KYMRIAH; Novartis Pharmaceuticals Corp.), an autologous anti-CD19 CAR-T cell containing the 4-1BB as co-stimulatory molecule.8,9 This approval was based on the results of the multicentric phase 2 study ELIANA, which demonstrated a MRD-negative CR in 81% of the pediatric and young adult patients treated and an overall survival (OS) of 76% at 12 months.10 With the development of new cellular therapy products involving other types of cells and/or target antigens, indications and patient eligibility for CAR-T cell treatments will be adapted for each new product in accordance with the results of clinical trials.7 The current scenario allows for some considerations:
- •
For tisagenlecleucel, the indication approved by the EMA includes pediatric and young adult patients up to 25 years of age with refractory B-ALL, relapsed after HSCT or in the second or subsequent relapse.11 Regarding the FDA, the approved indication is also for patients up to 25 years of age and refractory ALL or after the second relapse.12
- •
In the Brazilian scenario, the present panel of specialists points out that CAR-T cell therapy is currently recognized as a salvage therapy and, therefore, recommends that its indication should be for pediatric and young adult patients up to 25 years of age with refractory disease (primary or following the first relapse), in the second relapse, or relapsed after HSCT.
- •
The presence of leukemic infiltration in the central nervous system (CNS) does not contraindicate treatment with CAR-T cells.13,14 There is already data demonstrating the acceptable safety of the treatment with tisagenlecleucel in patients with secondary involvement of the CNS by lymphoma.15 However, this information does not appear in the FDA or EMA recommendations8,9 and patients with CNS infiltration should be followed closely after infusion, given the greater risk for neurotoxicity.16
- •
The expression of the target antigen (CD19) in the leukemic cell must be evaluated and confirmed by flow cytometry. Preferably, the antigen expression rates should be expressed as a percentage of total positive blasts. In the case of products involving other targets, the same rationale is applicable.
Treatment of R/R B-ALL in patients older than 26 years of age continues to be an unmet clinical need, given the absence of products commercially available at the moment of the elaboration of this consensus. The recent published results of the phase 2 international multicentric study ZUMA-3 evaluating the use of KTE-X19 (an autologous anti-CD19 CAR-T cell which contains the CD28 as co-stimulatory molecule recently approved for mantle cell lymphoma) in adults with R/R B-ALL signalizes that this product will be available for these patient population soon. The ZUMA-3 study included patients 18 years of age or older and demonstrated a CR or CR with incomplete hematological recovery (CRi) rate of 70.9%, with MRD-negative in 97% of the responding patients. The median OS was 18.2 months.17
Patient selection, logistics and pre-infusion evaluation and management before CAR-T cellsPre-apheresisThe decision to treat a patient with CAR-T cells should be coordinated by the clinical team responsible for the infusion/ treatment of the patient and the team manufacturing the CAR-T cells. In addition, specialized and multidisciplinary evaluation is imperative. The patient must have the minimum necessary clinical conditions to receive this high-cost therapy, avoiding its use in situations of short life expectancy. Additionally, most patients require bridging therapy to control the tumor burden during the period of manufacturing of the CAR-T cells, which also requires the coordinated efforts of the teams.
Once established the indication of the patient with B-ALL to receive anti-CD19 CAR-T cell therapy, it is important to define the approach to manage each of the phases of the process (apheresis, lymphodepletion and infusion of the CAR-T cells). At this moment, it is recommended, by well validated and defined operational procedures, that the following line of care be established:
- •
The referring physician must contact the cellular therapy team to discuss the patient eligibility to receive the treatment and to organize the logistics aspects. It is important to estimate the time between the apheresis and the receipt of the final product (CAR-T cells) by the center responsible for the infusion;
- •
At the same time, discuss the patient eligibility for bridging therapy to be initiated after the collection of T-cells by apheresis;
- •
The referral to the center for the collection and manufacturing of CAR-T cells should be performed by means of a specific form that contains the clinical and laboratory information relevant to the clinical condition of the patient.
The previous use of blinatumomab and inotuzumab ozogamicin should be discussed and considered in the treatment strategy of patients eligible for CAR-T cell therapy.
Blinatumomab is a bispecific antibody that recruits T-cells against CD19 positive cells with a mechanism of action similar to that of the anti-CD19 CAR-T cells. Thus, previous use of blinatumomab should be considered in the indication for CAR-T cell therapy due to reports of decreased CAR-T cell efficacy.18 In patients previously exposed to blinatumomab, it is important to check for the presence of a CD19-negative clone by flow cytometry, even before the indication for apheresis, which might be associated with escape after therapy with anti-CD19 CAR-T cells. In the case there is no expression of CD19 in the blasts, anti-CD19 CART-T should not be indicated.19 Decrease in the expression of CD19 in the blasts, following the use of blinatumomab, is associated with inferior outcomes of CAR-T cell therapy when compared to patients who had not used blinatumomab.18,20 Data on the previous use of inotuzumab ozogamicin indicate that its use may be associated with a lower expansion of CAR-T cells, however, this requires further validation.20 Currently, there is no anti-CD22 CAR-T cell product approved by any regulatory agency for use in clinical practice.21
In primarily refractory patients, i.e. those who fail to achieve MRD-negative CR, the sequential use of blinatumomab and CAR-T cells is not recommended, although frequently cannot be avoided. In the case of patients in relapse, blinatumomab should be avoided in the case that treatment with anti-CD19 CAR-T cells is being considered.
ApheresisThe evaluation of the number of circulating lymphocytes or T-cells is important for planning the apheresis (a minimum of 500 total lymphocytes/mm3 and/or 150 CD3+ lymphocytes/mm3 is recommended, although apheresis can be performed with lower counts).22,23 For patients previously submitted to HSCT, it is recommended the absence of acute or chronic graft versus host disease (GVHD) in activity before the apheresis and infusion of the CAR-T cells.24 A period without chemotherapy and immunosuppressants, variable according to the medication, is also recommended before apheresis and the infusion of the CAR-T cells (Table 1).25 Moreover, it is essential for the patient not to have an active infection before each stage of the treatment,26 as this increases the risk of contamination of the apheresis product and of CRS after infusion. Likewise, evaluation of comorbidities and toxicities in target organs (heart, kidneys, liver, and lungs) prior to CAR-T cell therapy is fundamental, as it can contraindicate the therapy.25 On the other hand, it is important to weigh the risk of toxicity related to the comorbidities versus the risk of alternate treatments for leukemia and the risk of CRS. A practical example is the presence of a moderate hepatic toxicity in a patient with a low leukemic burden. This patient will probably experience greater toxicity if he or she undergoes HSCT than that of being submitted to CAR-T cell therapy.
CAR-T: chimeric antigen receptor T cells, ALL: acute lymphoblastic leukemia, CRS: cytokine release syndrome, ICANS: immune effector cell-associated neurotoxicity syndrome, GVHD: graft versus host disease, DLI: donor lymphocyte infusion, G-CSF: granulocyte colony-stimulating factor, 6-MP: 6-mercaptopurine, 6-TG: 6-thioguanine, ATG: anti-thymocyte globulin
It is important to highlight that in the multicentric trial ELIANA10 the incidence of manufacturing failure, which is the failure of producing the CAR-T cell product, was approximately 7.5%. The patient and his or her family should be informed of this possibility.
Post-apheresisThe treatment with CAR-T cells for R/R ALL presents high rates of clinical response, with a prolonged period of remission in many patients. Different studies showed evidence of a correlation between low tumor burden prior to the infusion of CAR-T cells and decreased toxicity, with a better clinical response and OS. In a phase I study with 53 R/R ALL patients treated with anti-CD19-28z CAR-T cells, patients with a high tumor burden (≥ 5% of blasts in the bone marrow or extramedullary disease) had a higher incidence of CRS and neurotoxicity, and inferiorlong-term survival, when compared to patients with a low tumor burden.27 A retrospective study which included 15 institutions and 185 patients treated with tisagenlecleucel demonstrated that patients with a high tumor burden prior to the infusion of CAR-T cells had lower rates of complete response, OS and progression-free survival (PFS), when compared to patients with a low tumor burden or indetectable disease prior to the infusion.28
The manufacturing of the cells takes approximately four weeks, from the reception of the apheresis product to the release of the genetically modified cells. During this period, the patient is vulnerable to the progression of the disease and other complications.27,29 Chemotherapy schemes, known as “bridging therapies” can be used between the apheresis and the infusion of CAR-T cells.30 Perica et al. evaluated different strategies of bridging therapy and the impact on the outcome after anti-CD19-28z CAR-T cells for adult patients with ALL.31 The response to this treatment and a low tumor burden were correlated with favorable outcomes after the infusion of CAR-T cells. In addition, there was no significant difference in cell expansion in patients with persistent morphological disease, MRD-positive residual disease or in CR. There was no difference in the OS, CRS, and neurotoxicity grades 3 and 4 among patients who had received a chemotherapy scheme of high or low intensity, including monoclonal antibodies. However, bridging therapies based on high-intensity chemotherapy schemes were associated with higher rates of toxicity, such as severe infections (grades 3-4) and need for intensive clinical support in this period. There were no infectious complications in two patients who used blinatumomab and four patients on inotuzumab ozogamicin as bridging therapy. Of these six patients, three maintained complete remission following the treatment with CAR-T cells.31
Prior to the CAR-T cell infusion, patients should receive lymphodepletion chemotherapy, which provides a favorable environment for the maintenance and expansion of CAR-T cells. Studies have shown that the improvement in the CAR-T cell expansion with lymphodepleting chemotherapy may be related to the elimination of regulatory T-cells32 and to the increase in cytokines, such as IL-7 and IL-15.33-35 There are diverse lymphodepleting chemotherapy schemes, including cyclophosphamide, fludarabine, bendamustine, etoposide and corporal irradiation.34,36,37 In a study with 30 R/R ALL patients, it was observed that patients who received a combination of cyclophosphamide and fludarabine presented a greater expansion of cells and longer PFS compared to those who received cyclophosphamide alone or cyclophosphamide and etoposide.38 Currently, cyclophosphamide plus fludarabine is the most commonly used lymphodepletion scheme prior to the infusion of CAR-T cells for ALL and administered, most of the time, up to two days prior to the infusion of CAR-T cells.17,39
A summary of the recommendations for evaluation and selection for patients submitted to CAR-T cell therapy for ALL can be found in Table 1.
ALL management post-CAR-T cell infusionDetails of the clinical management post CAR-T cell infusion are addressed in the article I of the Consensus: “Structuring of centers for the clinical application and multidisciplinary management of patients submitted to CAR-T cell therapy”.40
Minimal residual diseaseThe routine follow-up after CAR-T cell infusion beyond 28 days should follow the same recommendations as those for the post-HSCT recommendations. It is recommended that a monthly evaluation of MRD be made up to 6 months following the infusion, every 3 months up to one year and every 6 months following that, or sooner in case of clinical alterations or changing in blood counts.41 In Philadelphia positive ALL, the tyrosine kinase inhibitor is initiated after day 40, although no clear benefit has been demonstrated as to the prophylactic use versus initiation in the presence of positive MRD.42,43
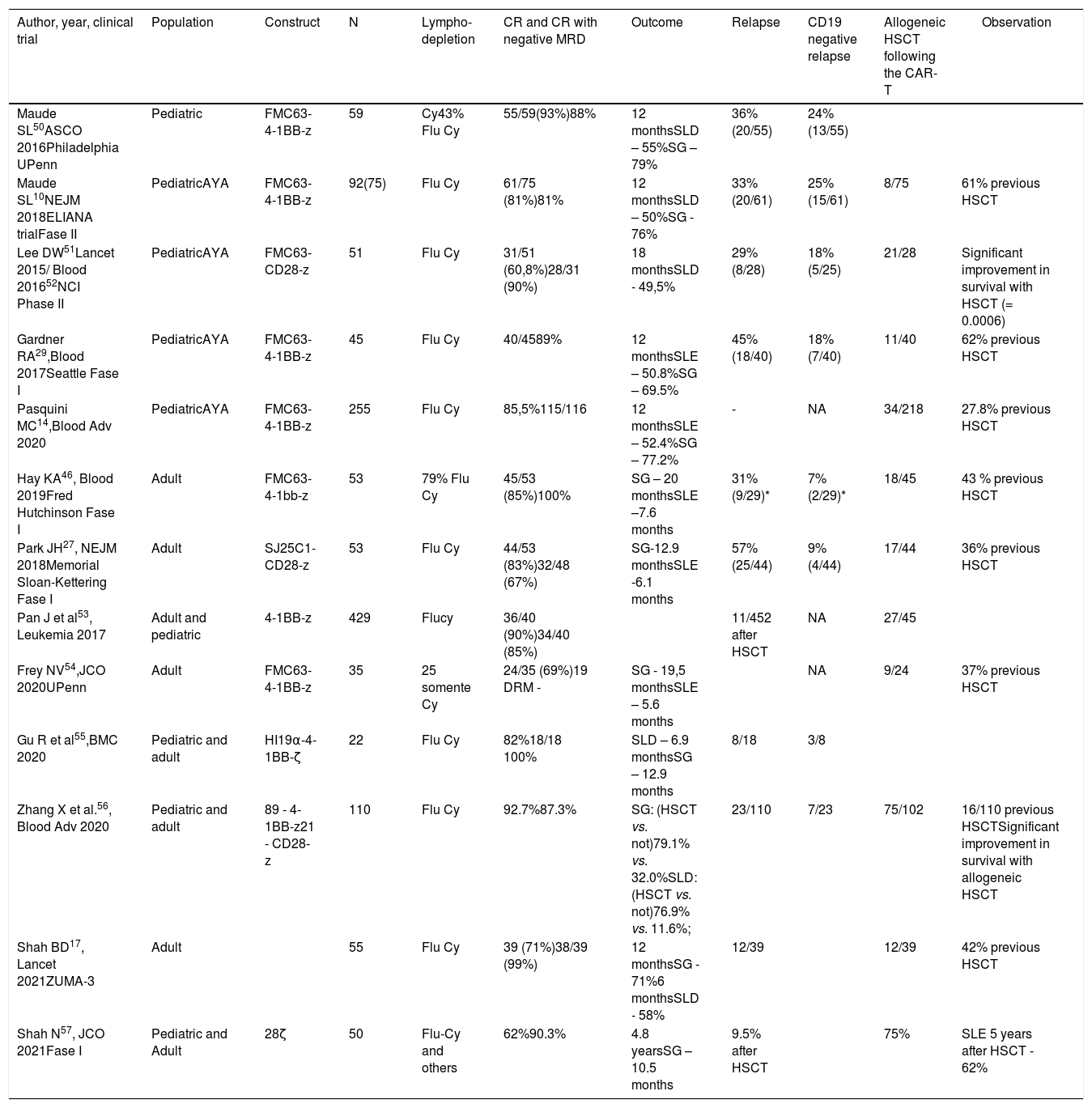
Consolidation with HSCTAlthough CAR-T cell therapy shows high rates of remission for R/R ALL in children and adults, a significant portion of patients will relapse after the therapy.44 Consolidation with HSCT has been used in some patients by different clinical trials following remission (Table 2). For tisagenlecleucel in children and young adults, few patients included in the ELIANA trial (8/75) were submitted to transplant after achieving CR.10 In the real-world experience with tisagenlecleucel, only 16% of the patients who achieved a CR with CAR-T cells underwent transplan, which indicates that it is possible to attain long-term remission without HSCT.14 On the other hand, in clinical trials with CAR-T cells in adults, the consolidation with HSCT is almost always recommended.27,45
Percentage of hematopoietic stem cell transplants following the CAR-T and CD19 negative relapses.
| Author, year, clinical trial | Population | Construct | N | Lympho-depletion | CR and CR with negative MRD | Outcome | Relapse | CD19 negative relapse | Allogeneic HSCT following the CAR-T | Observation |
|---|---|---|---|---|---|---|---|---|---|---|
| Maude SL50ASCO 2016Philadelphia UPenn | Pediatric | FMC63-4-1BB-z | 59 | Cy43% Flu Cy | 55/59(93%)88% | 12 monthsSLD – 55%SG – 79% | 36% (20/55) | 24% (13/55) | ||
| Maude SL10NEJM 2018ELIANA trialFase II | PediatricAYA | FMC63-4-1BB-z | 92(75) | Flu Cy | 61/75 (81%)81% | 12 monthsSLD – 50%SG - 76% | 33% (20/61) | 25% (15/61) | 8/75 | 61% previous HSCT |
| Lee DW51Lancet 2015/ Blood 201652NCI Phase II | PediatricAYA | FMC63-CD28-z | 51 | Flu Cy | 31/51 (60,8%)28/31 (90%) | 18 monthsSLD - 49,5% | 29% (8/28) | 18% (5/25) | 21/28 | Significant improvement in survival with HSCT (= 0.0006) |
| Gardner RA29,Blood 2017Seattle Fase I | PediatricAYA | FMC63-4-1BB-z | 45 | Flu Cy | 40/4589% | 12 monthsSLE – 50.8%SG – 69.5% | 45% (18/40) | 18% (7/40) | 11/40 | 62% previous HSCT |
| Pasquini MC14,Blood Adv 2020 | PediatricAYA | FMC63-4-1BB-z | 255 | Flu Cy | 85,5%115/116 | 12 monthsSLE – 52.4%SG – 77.2% | - | NA | 34/218 | 27.8% previous HSCT |
| Hay KA46, Blood 2019Fred Hutchinson Fase I | Adult | FMC63-4-1bb-z | 53 | 79% Flu Cy | 45/53 (85%)100% | SG – 20 monthsSLE –7.6 months | 31% (9/29)* | 7% (2/29)* | 18/45 | 43 % previous HSCT |
| Park JH27, NEJM 2018Memorial Sloan-Kettering Fase I | Adult | SJ25C1-CD28-z | 53 | Flu Cy | 44/53 (83%)32/48 (67%) | SG-12.9 monthsSLE -6.1 months | 57% (25/44) | 9% (4/44) | 17/44 | 36% previous HSCT |
| Pan J et al53, Leukemia 2017 | Adult and pediatric | 4-1BB-z | 429 | Flucy | 36/40 (90%)34/40 (85%) | 11/452 after HSCT | NA | 27/45 | ||
| Frey NV54,JCO 2020UPenn | Adult | FMC63-4-1BB-z | 35 | 25 somente Cy | 24/35 (69%)19 DRM - | SG - 19,5 monthsSLE – 5.6 months | NA | 9/24 | 37% previous HSCT | |
| Gu R et al55,BMC 2020 | Pediatric and adult | HI19α-4-1BB-ζ | 22 | Flu Cy | 82%18/18 100% | SLD – 6.9 monthsSG – 12.9 months | 8/18 | 3/8 | ||
| Zhang X et al.56, Blood Adv 2020 | Pediatric and adult | 89 - 4-1BB-z21 - CD28-z | 110 | Flu Cy | 92.7%87.3% | SG: (HSCT vs. not)79.1% vs. 32.0%SLD: (HSCT vs. not)76.9% vs. 11.6%; | 23/110 | 7/23 | 75/102 | 16/110 previous HSCTSignificant improvement in survival with allogeneic HSCT |
| Shah BD17, Lancet 2021ZUMA-3 | Adult | 55 | Flu Cy | 39 (71%)38/39 (99%) | 12 monthsSG - 71%6 monthsSLD - 58% | 12/39 | 12/39 | 42% previous HSCT | ||
| Shah N57, JCO 2021Fase I | Pediatric and Adult | 28ζ | 50 | Flu-Cy and others | 62%90.3% | 4.8 yearsSG – 10.5 months | 9.5% after HSCT | 75% | SLE 5 years after HSCT - 62% |
Factors related to a better event-free survival (EFS) in adults who had received CAR-T cells included lower tumor burden, low LDH (< 210 U/L) and higher platelet count (> 100,000/mL) prior to the therapy, lymphodepletion with fludarabine, MRD-negative CR, and persistent B-cell aplasia (< 0.01% CD19+ B-cells in peripheral blood) within 28 days.46 Other factors, such as an elevated number of regulatory T-lymphocytes and extramedullary diseases are related to a longer EFS and OS.47 The factors described can be considered when making the decision on the HSCT after CAR-T cell therapy in adults.
RelapseThere is a significant risk of relapse following CAR-T cell treatment for B-ALL. As previously described (see details in “Patient selection, pre-infusion logistics, evaluation and management before CAR-T cells – pre-apheresis”), in patients who had received tisagenlecleucel, previous exposure to blinatumomab and a high disease burden correlate with an increased risk for relapse. The relapses following anti-CD19 CAR-T can be divided according to the CD19 status.
The CD19-positive relapses generally occur early following the infusion and are related to poor expansion of CAR-T cells and/or a short term persistence.19 Factors related to this type of relapse are a lower disease burden prior to lymphodepletion and a rapid loss of B-cell aplasia following the infusion. The CD19-negative relapses are described as “selection by immunological pressure”19 and can occur at any moment following the CAR-T cell therapy. Various mechanisms can result in the expression of CD19 variants and immunological escape, such as the CD19 locus deletion, de novo frameshift and missense mutations, and lower expression of SRSF3.48 The risk factors for CD19-negative relapse include high tumor burden (DRM ≥ 10−2) pre-lymphodepletion and detectable MRD 4 weeks after the infusion.49
ConclusionAnti-CD19 CAR-T cell therapy is a very promising immunotherapy for the treatment of R/R ALL. The treatment has well-defined phases: collection of autologous lymphocytes, bridging therapy, manufacture of genetically modified lymphocytes, lymphodepletion therapy and, finally, the infusion of the CAR-T cells. It is important that the team be attentive to the medications contraindicated prior to the apheresis for the collection of lymphocytes and/or prior to the infusion of the CAR-T cells, while being trained to recognize and manage the toxicities specific to the treatment. In the post-treatment follow-up, the CAR-T cell therapy can be considered either a definitive therapy for R/R B-ALL or bridge to HSCT. The rigorous monitoring of B-cell aplasia and MRD following the infusion can assist the team in determining the best moment to indicate the HSCT with the disease still in remission.