Acute promyelocytic leukemia (APL) is one of the most incident hematologic neoplasms in the acute care setting.1
Treatment of APL consists of oral all-trans retinoic acid (ATRA), which has been extensively studied since 1990.1 The pharmacological target of ATRA is the Retinoic Acid Receptor-Alfa (RAR-¿) in malignant promyelocytes where it promotes their differentiation into mature myeloid lineages. Nowadays, 90% of patients achieve complete remission status in one to three months. Depending on risk classification, ATRA can be used as oral monotherapy, or combined with arsenic trioxide or anthracyclines.2
Despite the high rates of disease remission, APL is a medical emergency, as it causes a life-threatening coagulation disorder and respiratory insufficiency. The latter may be a result of differentiation syndrome (DS), which comprises 30% of all causes of deaths in the APL population.3
When patients are diagnosed with DS, they are often intubated to restore ventilation parameters. They also require the placement of an enteral tube to receive nutrients and medications.
So, how can we keep administering ATRA to these patients? In other words, the risk of occupational exposure to ATRA does not allow the nursing staff to manipulate such drugs by puncturing the capsule. Moreover, extracting the oily content from the hard-coated jelly capsules may lead to significant ATRA losses.4
In our institution, we have been admitting from two to eight patients per year with APL/DS to the emergency room and intensive care units. Because of the aforementioned ATRA handling problems, we have seen dose administration delays, unaware nursing technicians trying to manipulate ATRA (some of them in the fertile age) and pharmacy staff recurrently asking how to prepare this drug without losses and unnecessary occupational exposure. Unfortunately, there is not enough published information to support any kind of initiative, possibly due to the quick clinical evolution of APL and relatively low incidence in comparison to other neoplasms.5
In one of the most recent cases that we accompanied, we standardized a protocol to administer ATRA to intubated patients. A 22-year-old woman was admitted to the emergency room with dyspnea, dry cough and 85% oxygen saturation. She was diagnosed with high-risk PML and induction chemotherapy was promptly initiated: 50mg/m2 daunorubicin plus ATRA 45mg/m2/day. Two days later, her ventilatory parameters worsened due to DS, so she was intubated and an enteral tube was inserted. As DS is an ATRA-related life-threatening condition, the retinoid was suspended for two days until her respiratory parameters improved.
When ATRA was reintroduced, the problem of administering it by enteral tube arose, as her oral access was no longer available. After conducting a literature review, we suggest the following procedure (Figure 1):
- •
ATRA was dissolved in 10mL of distilled water (45°C), and 5mL of mineral oil was added to work as a lipophilic carrier. A 5-mL dead space was left inside a 20-mL syringe;
- ∘
We found that ATRA could be dissolved at higher temperatures without affecting its molecular structure.6–8 Mineral oil was chosen because the patient was already receiving it due to constipation secondary to narcotic agents;
- ∘
- •
The mixture was thoroughly shaken until the entire coat dissolved;
- ∘
Other authors have suggested that vigorous shaking should be applied to dissolve ATRA inside the syringe, which was observed by our pharmacy staff.9 It took more than 5min of shaking to dissolve the intact capsules completely;
- ∘
We also observed that cold temperatures did not remodel the carbohydrate polymer-based coat, so the solution maintained its colloidal physico-chemical properties;
- ∘
- •
All procedures were performed in a class II-B2 biological safety cabinet and the final pharmaceutical product was protected from light (Figure 1). The shelf life was set at 24h (extemporaneous solution) although, according to other authors, this can be extended6;
- •
We provided written and verbal instructions to nurses and physicians for clinical monitoring (liver enzymes and blood cell count, respectively for toxicity and efficacy) and safe ATRA handling (use of personal protection equipment and correct discard).
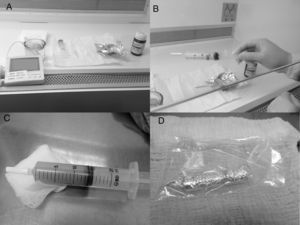
Process to manipulate all-trans retinoic acid (ATRA). (A) Materials: gown, gloves, class II-B2 biological safety cabinet, thermometer, 20-mL syringe, luer lock or other available syringe/lock system, light protection (e.g.: aluminum foil or other effective system), distilled water heated to 40°C and ATRA. (B) Syringe filled with 5–10mL of heated distilled water with ATRA and air space for effective mixing. (C) Final product after vigorous shaking. (D) ATRA protected from the light.
After seven days in the intensive care unit, the patient had improved clinically and she was weaned from mechanical ventilation and sedatives. She progressed to consolidation therapy, which consisted in mitoxantrone and oral ATRA, and was discharged from the oncology ward.
Due to the clinical benefits1–3,5 and physical and chemical stability,6 there were many advantages with the administration of ATRA dissolved in water through an enteral tube.
A Japanese group reported that a few patients who received ATRA by enteral tubes had significantly lower plasmatic levels of retinoic acid derivatives.10 However, two case reports discussed ATRA diluted in a manner similar to that described herein where patients achieved complete remission and it was suggested that the drug was absorbed in the gastrointestinal tract.9
It is worth remembering that, as a cytotoxic agent, nursing staff must not manipulate it without proper protection and materials and that uncoated ATRA is photosensitive.8 Thus, the disparities between the two aforementioned reports might be due to lack of protection against light, which explains why authors observed only physiological retinoid levels in their patients.6,9,10 The oral administration of ATRA as a single 80mg dose (∼45mg/m2 usual dosage) provides 347±266ng/mL of peak concentration in one to two hours.4,8 By looking at the pharmacokinetics graph of the authors, if an ATRA dose was entirely administered, we should observe at least a minimal peak concentration near drug administration, and logarithmic elimination of ATRA.10
Lastly, our experience is not absent of limitations and successful cases of ATRA administration through enteral tubes should be monitored regarding safety (control of the hypercoagulation state in APL, risk of bleeding and hepatotoxicity) and dosing requirements as different pharmacokinetic parameters may influence clinical response.9
ATRA is the mainstay of treating APL patients. Considering the benefits of maintaining retinoic-based therapy, the dissolving of coated capsules remains a valuable option for patients without oral access.
Conflicts of interestThere are no conflicts of interest.
On behalf of all Intensive Care Unit staff, we would like to thank Dr. Hipólito Carraro Jr. for his knowledge and outstanding clinical support to pharmacists, residents and patients.