The World Health Organization defines an adverse reaction as any harmful or undesirable and unintended response that occurs with the use of drugs in doses normally used. If not diagnosed early, especially in the elderly, it can be potentially fatal.
Decitabine is one of the therapeutic options for AML, both in monotherapy or in combination therapy for the treatment of elderly patients. Acute urinary retention has not yet been described in the literature as a side effect of decitabine.
We reported the case of an elderly female patient with acute myeloid leukemia treated with decitabine, who developed acute urinary retention, without any other justifiable cause besides the use of this medication.
Case descriptionA 74-year-old female patient was diagnosed with adverse risk acute myeloid leukemia (2017 European LeukemiaNet risk stratification), with RUNX1 mutation. At diagnosis, she presented 81.2% of myeloblasts in the myelogram, with expression of CD13, CD33, CD117, CD11c, CD38, CD123 in peripheral blood immunophenotyping.
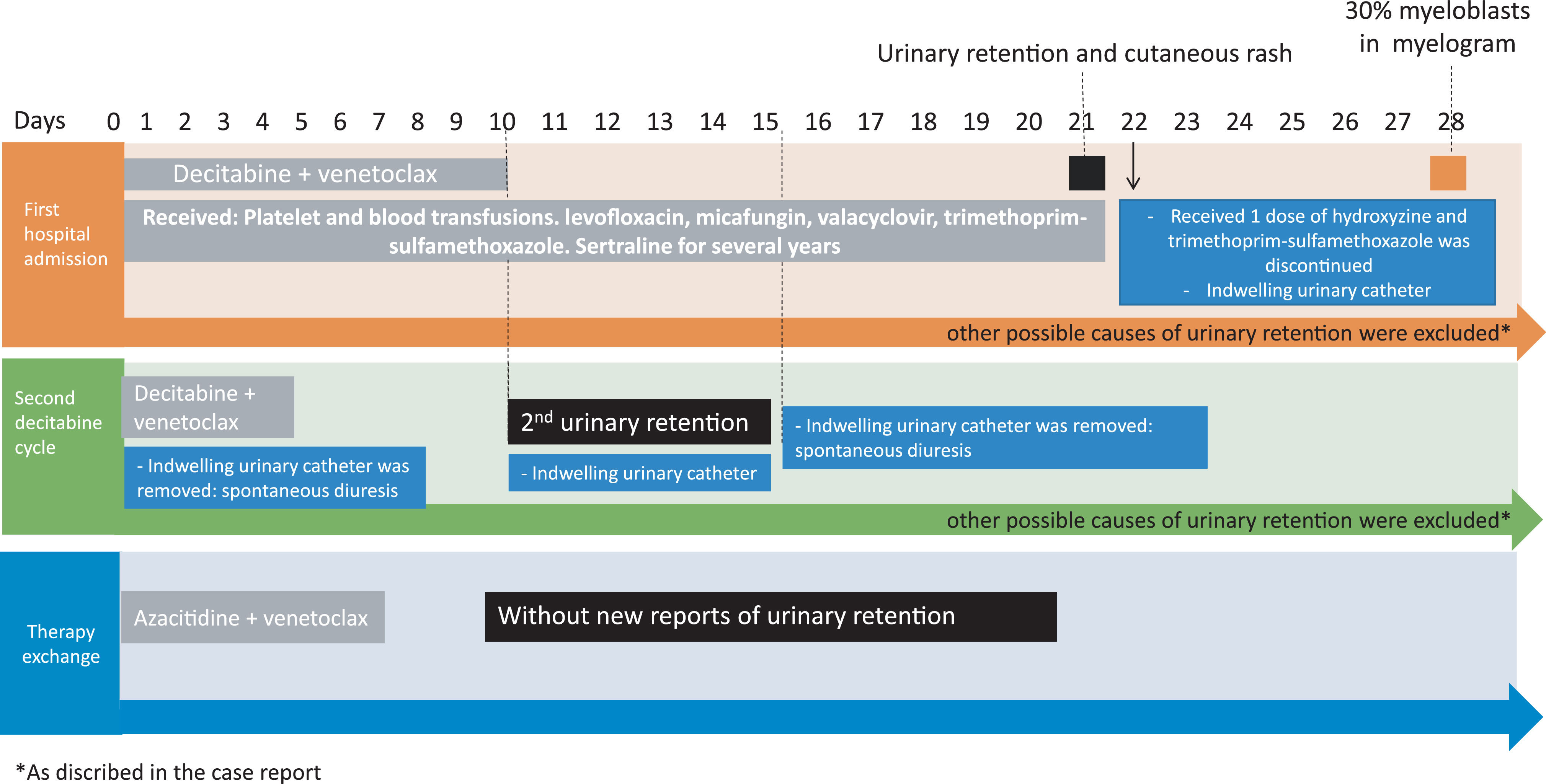
As she did not have an adequate performance status to undergo intensive chemotherapy, we preferred a therapeutic regimen with decitabine associated with venetoclax, as mentioned by Maiti et al.1 She then received 10 days of decitabine (20 mg/m²) and venetoclax (400 mg/day with daily dose escalation, 100200 400 mg/day).
Eleven days after the end of the decitabine infusion, she presented with urinary retention and intestinal constipation, requiring the passage of an indwelling urinary catheter and laxative agents. Renal function remained unchanged. Extensive urinary investigation was performed, with normal urinalysis and negative viral and bacterial tests. After seven days, indwelling urinary catheter was removed, with complete improvement in urinary retention, with no need for other invasive investigations, procedures or specific medications. During this period, she was evaluated by a urologist and no medication or mechanical changes that could cause such symptoms were identified.
During anamnesis, lower urinary tract and gynecological surgeries, obstetrical history, neurological disorders, diabetes, and trauma were excluded as possible causes of urinary retention. Physical examination did not show any pelvic organ prolapses. However, due to vulvovaginal atrophy because of age, the urethral meatus had significantly receded. Previously, the patient did not present significant lower urinary tract symptoms, and her American Urological Association Symptom Score was 5 (out of 35) with a qualityoflife index of 0 (delighted), in a scale from 0 to 6.
During treatment with decitabine, the patient received platelet and blood transfusions, but without the need for preparatory medications, such as diphenhydramine or hydrocortisone. During treatment with decitabine, the patient used levofloxacin 500 mg once daily, micafungin 100 mg once daily, valacyclovir 500 mg once daily and trimethoprimsulfamethoxazole 1 doublestrength tablet 3 times weekly for infectious prophylaxis.
After a cutaneous rash, when she received 1 dose of hydroxyzine, cotrimoxazole was discontinued at the first cycle. She had also been using sertraline for several years, without any adverse effects. Venetoclax was maintained throughout the period. The patient remained hospitalized throughout this period due to postchemotherapy bone marrow aplasia.
Twentyeight days after the first cycle of decitabine she had 30% myeloblasts in the myelogram and received a again the same hipometylating drug 20 mg/m2 for 5 days. There were no complications during the infusion days. Four days after the end of decitabine, the patient evolved again with urinary retention and intestinal constipation, without change the renal function or urine exams. No other medication had been started, other than the second cycle of decitabine. Again, the indwelling urinary catheter was introduced, with spontaneous improvement of symptoms after 5 days.
After the two episodes of urinary retention, during the two cycles of decitabine, it was decided to switch AML therapy to azacitidine associated with venetoclax, without new reports of urinary retention (Figure 1).
DiscussionAML is the most common acute leukemia in the elderly, with a mean age at diagnosis of 65 years.2–4 The prognosis of AML in elderly patients is generally poor, due to frequent cytogenetic abnormalities and poor performance status. Standard treatment is based on intensive chemotherapy for induction and consolidation with high doses of chemotherapy and/or hematopoietic stem cell transplantation, depending on the risk stratification. Despite this, many elderly patients with AML are considered ineligible for intensive treatment, due to important toxicity. For these patients, some less toxic therapeutic options are available aiming the improvement of quality of life, resolution of symptoms and increase of overall survival.
Decitabine is a hypomethylating agent, with a better profile of toxicity and, generally, well tolerated in frail elderly people diagnosed with AML. It can be administered as monotherapy or associated with other medications, such as venetoclax9-11. Venetoclax is an inhibitor of BCL2, which is an important protein in the survival and persistence of AML blasts, since it is a key regulator of the mitochondrial apoptotic pathway.5,6
Myelosuppression is the most common toxicity of decitabine and can cause, in particular, febrile neutropenia and fatigue.7,8 Gastrointestinal symptoms, such as the intestinal constipation reported in the case above, can be found in some cases. However, we did not find reports of urinary retention associated with the use of decitabine.
Acute urinary retention is defined as the inability to void urine, with a retained volume of urine of at least 200 ml and is a rare symptom in women. The main causal factor is bladder outlet obstruction, and in women, it is usually secondary to anatomical distortion, including pelvic organ prolapse and pelvic masses. As in this case, postmenopausal vaginal atrophy makes the urethra recedes and urethral catheterization may be challenging, requiring specific techniques. Nontheless, it is not a cause of obstruction. Neurological impairment or inefficient detrusor muscle can also be a cause for urinary retention.12-15 Hospitalized patients who develop acute urinary retention should be investigated for the use of medications, such as anticholinergics and sympathomimetics, and infections. We performed an extensive investigation for infection and use of other medication medication by the patient described here. In fact, the patient has use hydroxyzine by the time of the first retention. But since it was only one dose and it did not preclude the second retention, the only explanation for the urinary symptoms was the use of decitabine.
Urinary retention considerably increases the risk of urinary tract infection and impairment of renal function. It may also impact the quality of life of the patient, in special immunosuppressed ones and in treatment for hematological diseases, as in the case mentioned above.
ConclusionAdverse reactions to medication can be fatal for an elderly person undergoing treatment for acute myeloid leukemia (AML). This risk can be reduced with early diagnosis and discontinuation of the treatment. We report here the case of an elderly woman with AML who presented urinary retention associated with intestinal constipation as probable adverse reactions of decitabine.
Despite the necessity of further studies to investigate this association, clinicians have to be aware for the possibility of acute urinary retention in a patient using decitabine.