Relapse hampers successful hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) with disease relapse rates ranging from 20 to 50 % according to baseline disease risk group category among other characteristics.1 The approaches currently employed for the prevention and treatment of hematological relapse include the use of myeloablative conditioning, expedite tapering of immunosuppressants, preemptive donor lymphocyte infusion, FLT3-kinase inhibitors, hypomethylating agents, interferon-alfa, and immune checkpoint inhibitors.2 The hypomethylating agent azacitidine (AZA) when administered after engraftment improves graft-versus-leukemia effect by expanding effector cytotoxic CD8+T cell response.3-5 Additionally, it reportedly expands regulatory T cell (CD4+FoxP3) (particularly after six cycles of therapy), which seems to avoid overt graft-versus-host disease (GvHD).4 Furthermore, AZA is effective in recovering mixed donor chimerism and treatment of minimally measurable disease, as well as treating overt relapse.5,6 Here we report the experience of subcutaneous AZA as maintenance in a single HSCT center of Brazil.
Materials and methodsA retrospective cohort study was conducted at the Hospital Israelita Albert Einstein, São Paulo, SP, Brazil with over 18-year-old patients receiving their first allogeneic HSCT for either AML or high-risk MDS from 2011 to 2019. A careful review was made and data was collected regarding demographics (age at HSCT, sex, comorbidities), disease biology (karyotype, response to treatment, disease related index), patients overall health status (performance status measured using the Karnofsky-Lansky scale) and transplant strategy (donor type, conditioning regimen and graft source). The use of AZA after engraftment was recorded in the following manner: use as prophylaxis (for patients with no blasts in peripheral blood or no more than 4 % blasts in the bone marrow documented up to 30 days after transplant), use as treatment for overt relapse and no use at all. Moreover, duration of treatment, number of cycles, AZA dosage and route of administration, and reasons for eventual treatment discontinuation were also recorded.
Statistical analysisIn order to strengthen causal inference, a matched paired analysis (Group 1: AZA; Group 2: controls) was conducted using a propensity score as described by Randolph et al. 7. The variables of the propensity score utilized for matching were age, disease related index (categorized as ‘high-risk’ or ‘not high-risk’), conditioning intensity and Karnofsky Performance Score. Furthermore, a landmark analysis was established from 50 days post-transplant as a strategy to soften immortal time bias. The extracted outcomes of interest were overall survival and relapse.
Time to events were compared using Kaplan-Meier estimation or a cumulative incidence function, applying log rank and Gray's tests when appropriate as well as multivariate analysis. All statistical analysis were conducted with R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria); the packages ‘MatchIt’, ‘dplyr’, ‘survival’ and ‘cmprsk’ were used as needed. The project was submitted to and approved by the Institutional Board Ethics Committee (SPPG).
ResultsEighty-nine adult patients received their first allogeneic transplants for AML or high-risk MDS from 2011 to 2019: 55 patients were male, median age was 59 years old (range: 20–76 years), 39 patients received myeloablative conditioning, and donors were matched sibling donors (n = 29), matched unrelated donor (n = 37) and haploidentical donors (n = 23). Eight patients died prematurely and were, therefore, excluded from the landmark analysis; one patient was also excluded from analysis due to missing data regarding the circumstances surrounding AZA use. Thirty-two controls were selected after matching; the propensity score used was satisfactory after analyzing it using a graphical method (See Supplementary Figure S1. ‘Propensity Score Graphical Assessment’).8
The maintenance group (14 AML; 2 MDS) was characterized for patients at a high risk of relapse. Seven patients had an adverse risk karyotype at diagnosis (5 complex Karyotype, 1 with t(4;11), 1 with deletion of chromosome 5), also nine patients had either active disease at the time of transplant or positive MRD as detected by flow cytometry. These patients received a 5-day schedule of 32 mg/m2 AZA subcutaneously every 28 days, except for one patient who received a higher dose (75 mg/m2). The median time from graft infusion to AZA commencement was 47 days (interquartile range (IQR): 42–77), the median number of cycles was three (IQR: 2–12), and ten patients received up to four cycles of maintenance therapy.
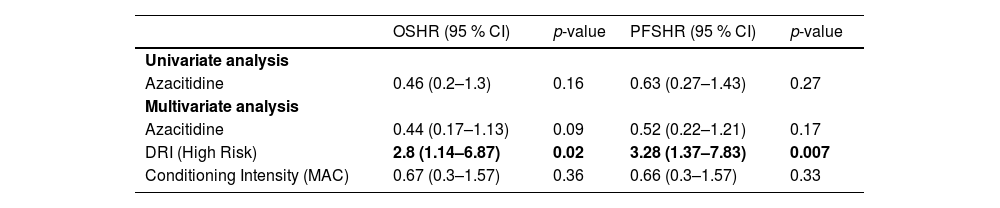
After a median follow-up time of 435 days (range: 51–2169) regarding this propensity score matched cohort, there were 24 deaths and 16 relapses. Overall survival was not improved with AZA maintenance therapy. The overall survival estimate two years after graft infusion was 41 % (95 % confidence interval - 95 % CI: 26.7–64.72) and 66.2 % (95 % CI: 45.5–96) for conventional therapy and AZA maintenance, respectively (Hazard ratio - HR = 0.52; 95 % CI: 0.26–1.915; p-value = 0.17) (Table 1). The main causes of death in this cohort were relapse (n = 9), infection (n = 7), GvHD (n = 7) and treatment toxicity (n = 1).
Cox Proportional hazards for overall survival and progression free survival.
OS, Overall survival; PFS, Progression free survival; HR, Hazard ratio; 95 % CI, 95 % confidence interval; DRI, Disease related index; MAC, Myeloablative conditioning regimen.
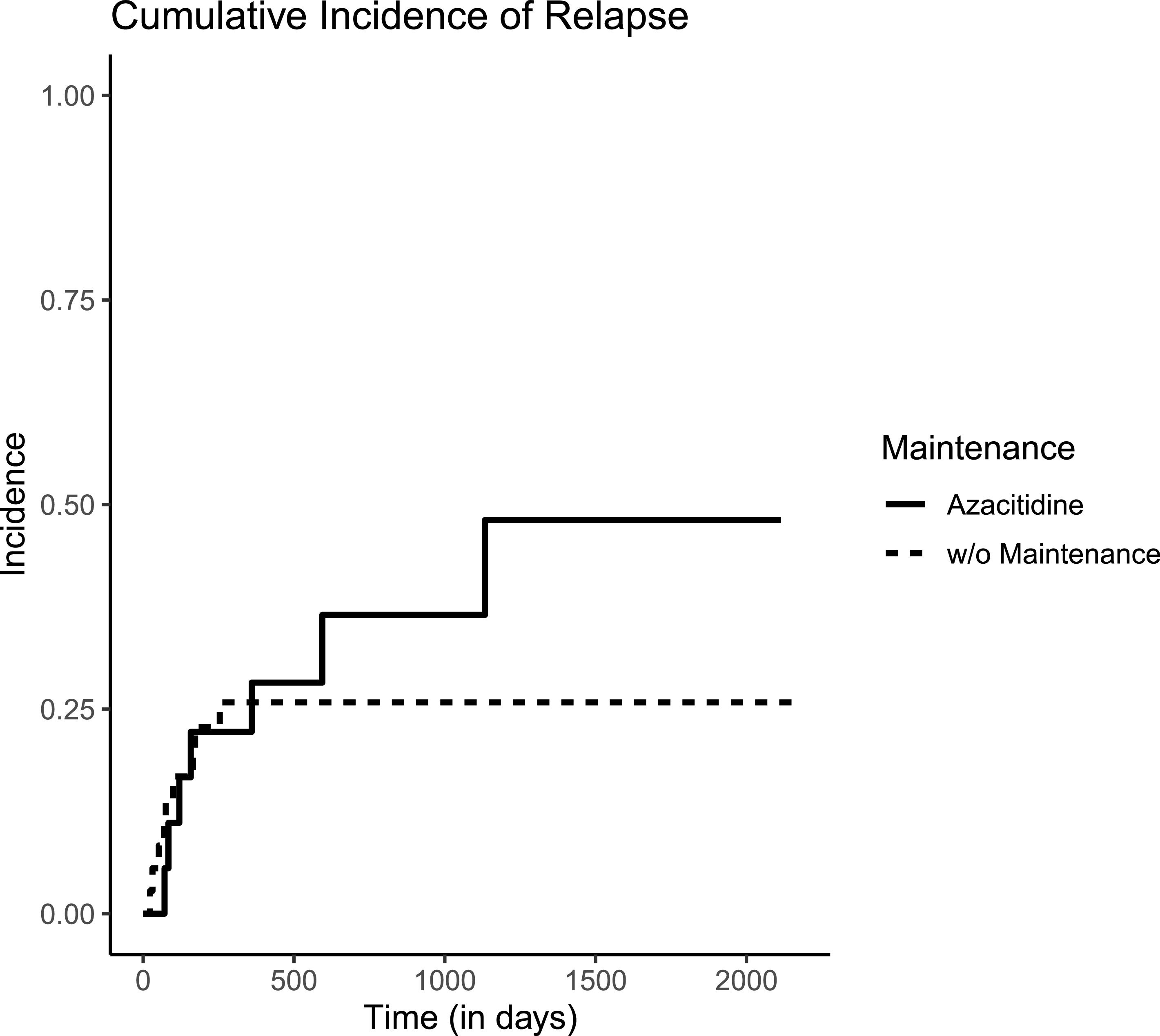
Additionally, relapse incidence was not reduced by subcutaneous AZA as shown in Figure 1. Cumulative incidence of relapse at two years of observation and AZA maintenance was 25.8 % versus 36.5 % (p-value for Gray's method = 0.5). Ten patients in the Control Group also received AZA but as treatment for overt relapse.
DiscussionMaintenance therapy after allogeneic transplant is a hot topic of discussion in the HSCT community. Relapses in this scenario forewarn a very grim prognosis, however questions related to patient selection, timing and duration of therapy as well as its impact on patients’ quality of life remain unanswered.8
Outcomes were not substantially improved by subcutaneous AZA maintenance in the present cohort. Among the limitations in the present report are the small sample size and the fact that it is a retrospective observational study. Even though a matched paired analysis was pursued, we are aware that it cannot prevent confounding as properly as randomized controlled trials.
This result concurs with the previous Phase 3 randomized trial conducted at MD Anderson Cancer Center.9 The aforementioned study, which compared AZA maintenance with observation, had issues such as the low rate of events in the control arm, slow accrual (which suggests selection bias), and difficulties in completing the planned maintenance schedule.9 These biases imply systematic and random biases that preclude further conclusions regarding maintenance from that set of data. On the other hand, a meta-analysis,10 that included 14 comparative studies evaluating either AZA or decitabine as treatment maintenance after allogeneic transplant, demonstrated improved outcomes for patients receiving hypomethylating agents. Also, a randomized controlled trial conducted in China by Gao et al.11 demonstrated meaningful improvements in relapse rates among high-risk transplanted patients that received low-dose decitabine instead of observation (HR: 0.32; 95 % CI: 0.18–0.57; p-value < 0.01).
Finally, AZA oral formulation (CC-486) showed promising results in a dose finding study for allogeneic transplant patients and in a randomized controlled trial of patients not submitted to allogeneic transplantation.4,12 The role of this agent after allogeneic stem cell transplantation is being evaluated in a randomized controlled trial versus placebo (Trial registration number: NCT04173533).