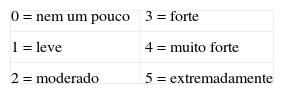the Blood Donation Reactions Inventory (BDRI) scale was proposed as part of a study about the predictors of psychological reactions in volunteer blood donors, as uncomfortable reactions are associated with a lower probability to return for further donations.
Objectiveto translate the Inventory into Brazilian Portuguese and evaluate its psychometric properties (validity and reliability). The inventory has 11 items, but the literature suggests that shorter inventories, of four or six items, should be used.
Methodsthis study was carried out at the blood center of Franca, Brazil. Three people with knowledge of English and familiarity with medical terms translated the Blood. Donation Reactions Inventory into Brazilian Portuguese. Aiming to evaluate the objectivity and relevance of the items of the translated instrument, its content was independently evaluated by a panel of eight assessors. After this, data on 1,001 blood donors was collected. Internal consistency was assessed by Cronbach’s alpha coefficient. An exploratory factor analysis with varimax rotation was used to analyze the measure for construct validity.
Resultsthe sample consisted of 65.8% men, and 27.3% first time donors. Internal consistency determined by Cronbach’s alpha coefficient was satisfactory for the 11, 6 and 4-item scales. Considering the factor analysis, the 11-item scale seems to measure more than one construct as three factors were identified with eigenvalues greater than 1. These factors correspond to ‘vasovagal adverse reactions’, ‘fear’ ‘anxiety/excitation’.
Conclusionthe Portuguese version of the Blood Donation Reactions Inventory is a valid and reliable instrument for collecting information regarding systemic reactions experienced by blood donors. The 6-item scale seems to be useful when the objective is to measure only vasovagal adverse reactions.
© 2014 Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. All rights reserved.
The maintenance of blood component stocks is a constant challenge for blood transfusion services. Recruitment strategies of blood donors must aim to ensure the necessary number of donors considering seasonal variations in the number of donations,1 and to focus on the quality of the material collected. In this context, the search for repeat blood donors is essential, as those tested and retested donors provide blood bags with a greater safety margin for the recipient, a smaller number of discarded blood components,2 and a lower number of positive test results in the screening for infectious diseases.
The loyalty of blood donors can be enhanced using strategies that increase donation accessibility,3 training the professionals at the blood centers,4 and fulfilling the expectations and increasing the satisfaction of the donors regarding the service provided.4-7 The literature shows that factors associated with the return of a blood donor for further donations are Rh factor,8 age,8,9 education,8-10 and gender.9 In addition, some authors have also highlighted the need to pay attention to the physical effects of blood donation,10 as uncomfortable reactions are associated with a lower probability to return for further donations.11,12 Among these reactions are fainting, vertigo and dizziness.
The Blood Donation Reactions Inventory (BDRI) scale was proposed as part of a study about predictors of psychological reactions in volunteer blood donors.13 The scale consists of 11 items, each corresponding to a reaction or feeling regarding the latest blood donation: (1) faintness (such as feeling faint or losing consciousness), (2) dizziness, (3) weakness, (4) facial flush, (5) visual disturbance (such as blurred vision or tunnel vision), (6) difficulty hearing, (7) lightheadedness, (8) rapid or pounding heartbeat, (9) sweating, (10) rapid or difficult breathing and (11) nausea or upset stomach. Donors answer questions on these items after a donation using the 6-point Likert scale ranging from 0 (‘not at all’) to 5 (‘to an extreme degree’). Thus, higher values are associated with greater reaction intensity. The responses are summed to the final score producing values between 0 and 55.
The psychometric properties of BDRI were evaluated by France et al.,14 who found high internal consistency for the scale, concurrent validity with other measures of reactions to blood donation and construct validity supported by a factor analysis. This study further showed that an abbreviated version of the BDRI containing only items 1, 2, 3 and 7 of the original instrument would have good psychometric properties and could replace the original scale of 11 items. France et al. also concluded that BDRI is brief, easily understood by donors, and quick to administer and score.14
In another study, France et al.15 showed that high BDRI scores are associated with a significant reduction in the probability of return of blood donors, suggesting that the instrument is an effective tool for predicting whether a donor will make further donations.
Routinely, the blood donors at the Regional Blood Center (Hemocentro) of Ribeirão Preto, Brazil, and at its satellite units, are observed during donation by a nurse who provides additional care to those who experience an adverse reaction or accident during blood collection. When a blood donor presents an adverse reaction, the nurse completes a standard form entitled ‘Notice of Adverse Reaction during Donation’ (NARD). The nurse writes on this form which systemic reactions were experienced, their intensity, vital signs and other signs and symptoms, including possible incidents during blood collection. After completion, the nurse is responsible for adding data to the donor’s record so that professionals performing the screening of future donations are informed about the incident. The translated version of BDRI does not intend to replace the NARD because its purpose is different. While the NARD allows routine monitoring of adverse reactions, the BDRI is useful for studies that evaluate reactions aimed to, for example, establish strategies to increase blood donor satisfaction and retention. In this context, France et al.15 cite some disadvantages of the measures based on the observations of the professionals responsible for blood collection in predicting the return of the donor. These professionals may not be able to detect subjective symptoms such as dizziness, vertigo, stress, nervousness or even excessive distress of the person who has a sharp object in his/her arm, and consequently cannot assess the intensity of the donors’ reactions. This means that a ‘slight’ sensation perceived by the nurse may be so uncomfortable for the donor as to influence his/her decision not to return for further donations. Given that the BDRI assesses the experience from the perspective of the donor, it is more sensitive to the intensity of the reaction.
Thus, the objective of this study was to translate the BDRI into Brazilian Portuguese and study internal consistency, criteria, and construct validation of the translation. In this study, the 11-item version of the BDRI and the abbreviated 6-and 4-item versions proposed by France et al.14 will be considered. The 6-item scale considers items 1, 2, 3, 5, 7 and 11 of the original instrument with the respective total score ranging from 0 to 30 points. The 4-item scale considers items 1, 2, 3 and 7 with the respective total score ranging from 0 to 20 points.
MethodsTranslationFirst, three people with knowledge of English and familiarity with medical terms independently worked on the translation of the BDRI scale. It was intended that the meaning of the 11 terms describing the feelings and reactions of each item of the instrument had a literal correspondence between the original English version and the Portuguese translation. This resulted in three different versions, which were later compared in order to produce a final consensual version considering cultural aspects of the target population. Thus, the relevance, appropriateness, and acceptability of the style employed were reevaluated given that the educational and socioeconomic levels are highly variable in the population for which the instrument is intended.
Content validationAiming to assess the objectivity and relevance of the items of the translated instrument, its content was independently evaluated by a panel of eight qualified assessors.16 All evaluators were nurses with considerable experience in the field of transfusion medicine with at least one year of practice in collecting blood components and complete higher education. Preference was given to professionals with doctorate or master degrees, with published papers related to the theme of blood donation. The evaluators were informed about the purposes of the study and asked to classify as valid or not each one of the items of the instrument. Thus, the content validity index (CVI) was calculated for each item.17 The items translated with a CVI of 100% were kept for the definitive instrument. The translations of items with a CVI less than 100% and greater than or equal to 80% were discussed, and the translation of items with a CVI of less than 80% were changed.
Data collectionOnce the content was validated, the instrument was applied to a sample of 1,001 consecutive blood donors at the Blood Center in Franca (northeastern Sao Paulo State). The determination of sample size was based on statistical considerations as presented by Schmidt et al.,18 who reported the minimum number of subjects needed to validate studies.
It was considered that all individuals donating blood at the time of data collection would be able to participate in the study regardless of their literacy, since the data was collected by interviews. Therefore, the inclusion criteria for this study are the same as those in force in Brazil regarding individuals who are able to donate blood: age between 18 and 65 years, weighing more than 50 kilograms and being in good health. The exclusion criteria are naturally the following: being pregnant, donors known to have had Chagas disease, malaria (or have been in an endemic area), hepatitis after 10 years of age, leprosy, AIDS, diabetes, cancer, illicit drug use or sexual risk behavior.
Data collection was performed by four nurses with experience in blood collection for over a year. Blood donors were invited to participate voluntarily in the study after being informed of its purpose. Furthermore it was explained that all the information would be kept anonymous. Consent was established in the blood collection room, immediately after blood donation, with the donors signing an informed consent form. Despite the large sample size, no blood donor refused to participate in the study.
Shortly after the informed consent form was signed, each blood donor was interviewed and asked to complete a structured questionnaire containing questions regarding age, gender, number of previous blood donations and level of education based on the translated version of the BDRI. The interviews were conducted individually in an appropriate place so that the interviewer could read each item to the donor as well as the contents in parentheses (see Appendix A) and, if necessary, discuss the meaning of each item. However, no additional explanation was given about the items. During data collection, modification of the blood donors’ routine was prevented in order to avoid affecting the flow of service.
Internal consistencyThe internal consistency of the translated instrument was assessed using Cronbach’s alpha coefficient.19 This coefficient is a value ranging between 0 and 1 that indicates whether the instrument items form a consistent set in order to measure the same object. The higher the coefficient, the higher the consistency.20 The item-scale correlation coefficients were also calculated to evaluate the correlation between individual items and the BDRI scale. Cronbach’s alpha coefficient was calculated by removing respective items from the BDRI and identifying the likely impact of the removal.21 When it is noted that the elimination of any of the items does not imply any significant change in the previously calculated Cronbach’s alpha coefficient, it is evidence that the item is not measuring an aspect related to the assessed object. Confidence intervals (CI) for the Cronbach’s alpha coefficient were obtained using the approach published by Bonett.22
Criterion validityThe validity of criteria was achieved by comparing the scores obtained from the BDRI and the number of signs and symptoms observed by the nurse during blood donation reported in the NARD. The agreement between BDRI scores, rated as 0 or 1 point or more, and reports of signs and symptoms were measured using the kappa coefficient23 with its respective 95% CI.
Construct validityConstruct validity was determined by examining the factor structure of the translated BDRI. Thus, an exploratory factor analysis with varimax rotation was used to value the dimensionality of the translated instrument. The suitability of a one-factor solution was assessed using scree plots (graphs not showed here) and evaluating how many factors had eigenvalues greater than 1. Kaiser’s measure of sampling adequacy (MSA)24,25 was used to examine data adequacy for factor analysis, where values greater than 0.8 are considered good. Communalities were used to estimate the amount of variance accounted for by the factor solution for each variable.
Ethical considerationsEthical approval was obtained from the Research Ethics Committee of the Medical School in Ribeirão Preto, Universidade de São Paulo. Informed consent was obtained from the blood donors before their entry in the study.
Statistical analysisStatistical analyses were performed using the SAS version 9 and the R software.
ResultsAfter the translation process, the Portuguese version of the BDRI was evaluated by the panel of evaluators and the CVI was generated for each item in order to assess the content validity. Eight items were classified as potentially valid by all eight evaluators (CVI equal to 100%) and were included in the final inventory. Another three items received suggestions for small changes by the assessors (CVI=87.5%), these suggestions were discussed until a definitive version of the translated BDRI was obtained.
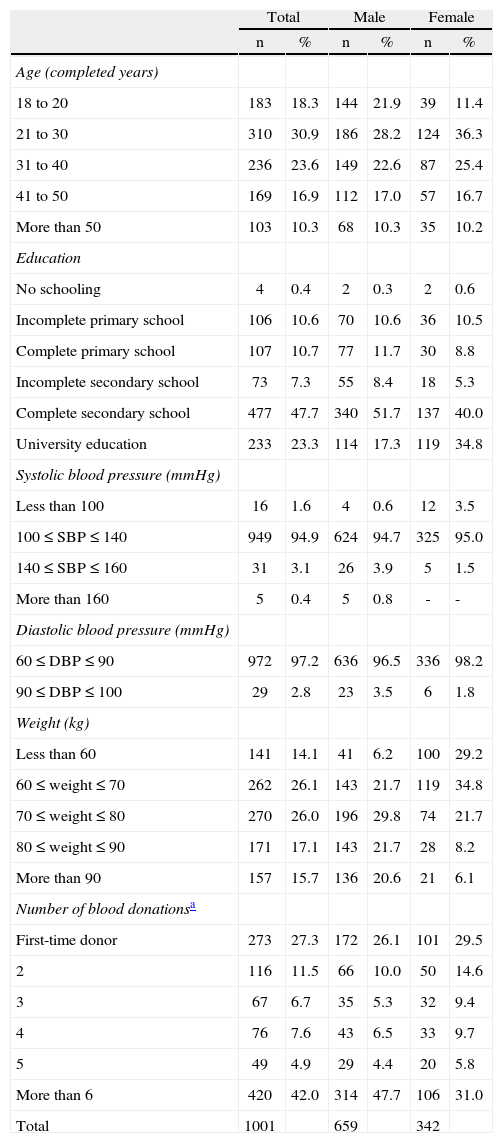
Next, the BDRI was administered to the sample of 1,001 blood donors. Table 1 shows the characteristics of the study sample. Of the donors, 65.8% were male, 30.9% were in the age group of 21-30 years and 27.3% were first-time donors. The final Portuguese version of the BDRI instrument is shown in Appendix A.
- Characteristics of the participants in this study.
| Total | Male | Female | ||||
| n | % | n | % | n | % | |
| Age (completed years) | ||||||
| 18 to 20 | 183 | 18.3 | 144 | 21.9 | 39 | 11.4 |
| 21 to 30 | 310 | 30.9 | 186 | 28.2 | 124 | 36.3 |
| 31 to 40 | 236 | 23.6 | 149 | 22.6 | 87 | 25.4 |
| 41 to 50 | 169 | 16.9 | 112 | 17.0 | 57 | 16.7 |
| More than 50 | 103 | 10.3 | 68 | 10.3 | 35 | 10.2 |
| Education | ||||||
| No schooling | 4 | 0.4 | 2 | 0.3 | 2 | 0.6 |
| Incomplete primary school | 106 | 10.6 | 70 | 10.6 | 36 | 10.5 |
| Complete primary school | 107 | 10.7 | 77 | 11.7 | 30 | 8.8 |
| Incomplete secondary school | 73 | 7.3 | 55 | 8.4 | 18 | 5.3 |
| Complete secondary school | 477 | 47.7 | 340 | 51.7 | 137 | 40.0 |
| University education | 233 | 23.3 | 114 | 17.3 | 119 | 34.8 |
| Systolic blood pressure (mmHg) | ||||||
| Less than 100 | 16 | 1.6 | 4 | 0.6 | 12 | 3.5 |
| 100≤SBP≤140 | 949 | 94.9 | 624 | 94.7 | 325 | 95.0 |
| 140≤SBP≤160 | 31 | 3.1 | 26 | 3.9 | 5 | 1.5 |
| More than 160 | 5 | 0.4 | 5 | 0.8 | - | - |
| Diastolic blood pressure (mmHg) | ||||||
| 60≤DBP≤90 | 972 | 97.2 | 636 | 96.5 | 336 | 98.2 |
| 90≤DBP≤100 | 29 | 2.8 | 23 | 3.5 | 6 | 1.8 |
| Weight (kg) | ||||||
| Less than 60 | 141 | 14.1 | 41 | 6.2 | 100 | 29.2 |
| 60≤weight≤70 | 262 | 26.1 | 143 | 21.7 | 119 | 34.8 |
| 70≤weight≤80 | 270 | 26.0 | 196 | 29.8 | 74 | 21.7 |
| 80≤weight≤90 | 171 | 17.1 | 143 | 21.7 | 28 | 8.2 |
| More than 90 | 157 | 15.7 | 136 | 20.6 | 21 | 6.1 |
| Number of blood donationsa | ||||||
| First-time donor | 273 | 27.3 | 172 | 26.1 | 101 | 29.5 |
| 2 | 116 | 11.5 | 66 | 10.0 | 50 | 14.6 |
| 3 | 67 | 6.7 | 35 | 5.3 | 32 | 9.4 |
| 4 | 76 | 7.6 | 43 | 6.5 | 33 | 9.7 |
| 5 | 49 | 4.9 | 29 | 4.4 | 20 | 5.8 |
| More than 6 | 420 | 42.0 | 314 | 47.7 | 106 | 31.0 |
| Total | 1001 | 659 | 342 | |||
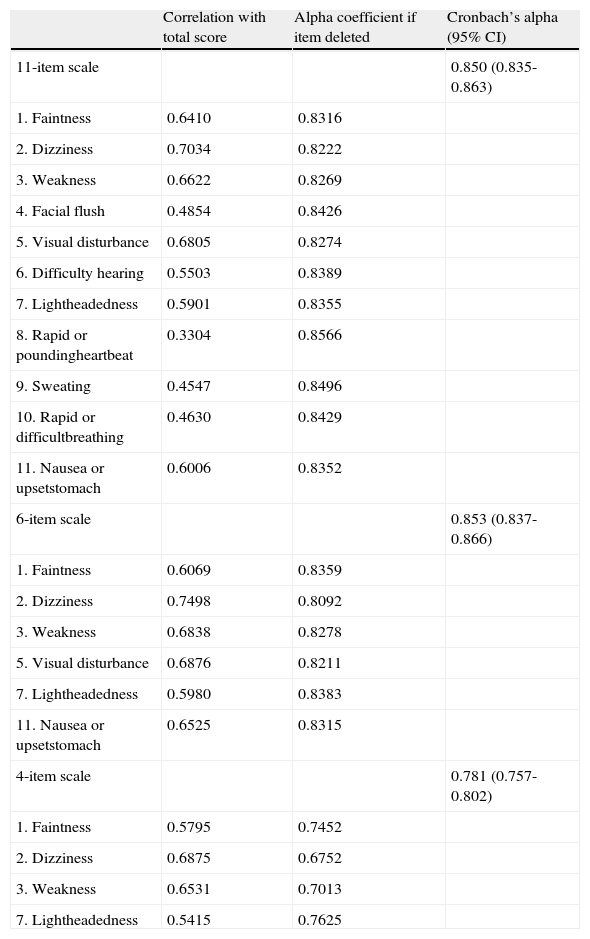
Table 2 shows the analysis of the internal consistency of the Portuguese version regarding the 11-, 6-and 4-item scales. The internal consistency determined by Cronbach’s alpha coefficient was satisfactory for all three scales. Cronbach’s alpha coefficient was estimated as 0.850 for the 11-item scale, 853 for the 6-item scale, and 0.781 for the 4-item scale. The alpha coefficient for deleted items showed that removing any single item would not yield a higher result. Therefore, considering all the three scales, the items are related enough to be combined into a single construct. It can also be noted in Table 2 that, when considered the 11-item scale, the correlation with total score are equal or less than 0.55 for items 4, 6, 8, 9 and 10. These items are excluded in the 6-item scale.
- Internal consistency analysis of the Portuguese version of the Blood Donation Reactions Inventory (BDRI) for the 11, 6 and 4-item scales.
| Correlation with total score | Alpha coefficient if item deleted | Cronbach’s alpha (95% CI) | |
| 11-item scale | 0.850 (0.835-0.863) | ||
| 1. Faintness | 0.6410 | 0.8316 | |
| 2. Dizziness | 0.7034 | 0.8222 | |
| 3. Weakness | 0.6622 | 0.8269 | |
| 4. Facial flush | 0.4854 | 0.8426 | |
| 5. Visual disturbance | 0.6805 | 0.8274 | |
| 6. Difficulty hearing | 0.5503 | 0.8389 | |
| 7. Lightheadedness | 0.5901 | 0.8355 | |
| 8. Rapid or poundingheartbeat | 0.3304 | 0.8566 | |
| 9. Sweating | 0.4547 | 0.8496 | |
| 10. Rapid or difficultbreathing | 0.4630 | 0.8429 | |
| 11. Nausea or upsetstomach | 0.6006 | 0.8352 | |
| 6-item scale | 0.853 (0.837-0.866) | ||
| 1. Faintness | 0.6069 | 0.8359 | |
| 2. Dizziness | 0.7498 | 0.8092 | |
| 3. Weakness | 0.6838 | 0.8278 | |
| 5. Visual disturbance | 0.6876 | 0.8211 | |
| 7. Lightheadedness | 0.5980 | 0.8383 | |
| 11. Nausea or upsetstomach | 0.6525 | 0.8315 | |
| 4-item scale | 0.781 (0.757-0.802) | ||
| 1. Faintness | 0.5795 | 0.7452 | |
| 2. Dizziness | 0.6875 | 0.6752 | |
| 3. Weakness | 0.6531 | 0.7013 | |
| 7. Lightheadedness | 0.5415 | 0.7625 |
95% CI: 95% confidence interval
The criterion validity analysis was performed comparing BDRI scores and presence or absence of signs and symptoms observed by the nurse during the donation, as reported in the NARD. Table 3 describes the results of this comparison, considering the 11, 6 and 4-item BDRI scales. All of the Kappa coefficients were relatively low suggesting a poor overall agreement between the two methods. However, only 12 blood donors had reported signs and symptoms from the NARD, whereas a much higher number reported at least one reaction using the BDRI (243 donors considering the 11-item scale). Of the 12 donors with signs and symptoms according to the NARD, 11 (91.7%) described a reaction when the 11-item BDRI was used and 10 (83.3%) described a reaction when the 6 and 4-item scales were used (Table 3). In addition, of the 989 donors without signs and symptoms as reported by the NARD, expressive proportions did not describe any reaction in the 11, 6 and 4-item BDRI scales (respectively, 76.5%, 86.7% and 87.2%). These results show that the BDRI is more sensitive than the NARD to identify the presence of blood donation reactions. As the translated BDRI was able to identify most of the signs and symptoms reported in the NARD, the results suggest reasonable criteria validity for the Portuguese version of the BDRI despite the low kappa coefficients.
- Comparison between reports of signs and symptoms from the Notice of Adverse Reaction during Donation (NARD) and Blood Donation Reactions Inventory (BDRI) scores rated as 0 points or 1 or more points.
| BDRIscores | Points | Reports of signs and symptoms from NARD | Total | Kappa coefficient (95% CI) | |
| Present | Absent | ||||
| 11-items | 0 | 1 (8.3%) | 757 (76.5%) | 758 | 0.065 (0.02-0.10) |
| 1 or more | 11 (91.7%) | 232 (23.5%) | 243 | ||
| 6-items | 0 | 2 (16.7%) | 857 (86.7%) | 859 | 0.110 (0.04-0.18) |
| 1 or more | 10 (83.3%) | 132 (13.3%) | 142 | ||
| 4-items | 0 | 2 (16.7%) | 862 (87.2%) | 864 | 0.115 (0.04-0.18) |
| 1 or more | 10 (83.3%) | 127 (12.8%) | 137 | ||
| Total | 12 (100.0%) | 989 (100.0%) | 1001 | ||
Table 4 presents the results of the exploratory factor analysis. Factor loadings with values greater than 0.5 indicating items that were better characterized by the factors. The 11-item scale seems to measure more than one construct as three factors have eigenvalues greater than 1. Factor 1 is better correlated to items 1, 2, 3, 5, 6, 7 and 11 (factor loadings over 0.5). Factor 2 correlates better with items 1, 4, 6 and 9, and factor 3 correlates better with items 8 and 10. Kaiser’s MSA was 0.86 for the 11-point scale, which indicates that the data was well suited for factor analysis. The 6- and 4-item scales measure only one construct as only the first eigenvalue is greater than 1. In these cases, all factor loadings are positive and greater than 0.5. In all factor analyses, examination of communality magnitudes showed that the factors accounted for a considerable proportion of the variance in all items.
- Exploratory factor analysis of the Portuguese version of the Blood Donation Reactions Inventory (BDRI) for the 11-, 6- and 4-item scales.
| BDRI item | Factor pattern (varimax rotation) | Communality | ||
| Factor 1 | Factor 1 | Factor 3 | ||
| 11-item scale | ||||
| 1. Faintness | 0.5282 | 0.5628 | 0.0643 | 0.60 |
| 2. Dizziness | 0.7932 | 0.1778 | 0.2331 | 0.72 |
| 3. Weakness | 0.7475 | 0.1408 | 0.2860 | 0.66 |
| 4. Facial flush | 0.1128 | 0.7994 | 0.1814 | 0.68 |
| 5. Visual disturbance | 0.6969 | 0.4273 | 0.0174 | 0.67 |
| 6. Difficulty hearing | 0.5332 | 0.5495 | -0.1151 | 0.60 |
| 7. Lightheadedness | 0.6901 | 0.3213 | -0.0058 | 0.58 |
| 8. Rapid or pounding heartbeat | 0.0852 | 0.1280 | 0.8272 | 0.71 |
| 9. Sweating | 0.1343 | 0.7120 | 0.2062 | 0.57 |
| 10. Rapid or difficult breathing | 0.2442 | 0.1499 | 0.7662 | 0.67 |
| 11. Nausea or upset stomach | 0.7728 | -0.0188 | 0.2679 | 0.67 |
| Eigenvalue | 4.818 | 1.233 | 1.072 | |
| Contribution to the total variation | 43.8% | 11.2% | 9.8% | |
| Variance explained by each factor | 3.409 | 2.142 | 1.573 | |
| Kaiser’s MSA=0.860 | ||||
| 6-item scale | ||||
| 1. Faintness | 0.7258 | 0.53 | ||
| 2. Dizziness | 0.8447 | 0.71 | ||
| 3. Weakness | 0.7912 | 0.63 | ||
| 5. Visual disturbance | 0.7924 | 0.63 | ||
| 7. Lightheadedness | 0.7294 | 0.53 | ||
| 11. Nausea or upset stomach | 0.7546 | 0.57 | ||
| Eigenvalue | 3.596 | |||
| Contribution to the total variation | 59.9% | |||
| Variance explained by each factor | 3.596 | |||
| Kaiser’s MSA=0.834 | ||||
| 4-item scale | ||||
| 1. Faintness | 0.7640 | 0.58 | ||
| 2. Dizziness | 0.8411 | 0.71 | ||
| 3. Weakness | 0.8147 | 0.66 | ||
| 7. Lightheadedness | 0.7330 | 0.54 | ||
| Eigenvalue | 2.492 | |||
| Contribution to the total variation | 62.3% | |||
| Variance explained by each factor | 2.492 | |||
| Kaiser’s MSA=0.785 | ||||
MSA: measure of sampling adequacy.
Many studies have shown that the experience of adverse events such as vasovagal reactions can decrease the retention of blood donors.26-29 Thus, strategies aimed to know how blood donors react to donation and how the risks of adverse reactions can be minimized are of great importance to maintain the blood supply. The BDRI can be a very useful research tool about attitudes towards blood donation and to plan measures to promote satisfaction and retention of blood donors. In the present study, the BDRI was translated into Portuguese; this version is a valid and reliable instrument to collect information regarding systemic reactions experienced by blood donors.
The internal consistency analysis showed similar Cronbach’s alpha coefficient estimates for the 11- and 6-item scales (Table 2). The coefficient was lower for the 4-item scale. In addition, the items with lower factor loadings in the 11- item scale are those that do not appear in the 6-item scale. These results suggest that the use of the 4-item scale may cause a loss of some information to measure a given object, whereas the synthetic 6-item scale measures the same object as good as that of 11 items. On the other hand, the decision to use the 11- or 6-item scale demands a greater understanding of the composition of each one, which is assisted by the construct validity.
Considering the 11-item scale, the results of the exploratory factor analysis shown in Table 4 suggest that Factor 1 is better correlated to items that correspond to vasovagal adverse reactions on blood donation in response to bleeding (Items 1, 2, 3, 5, 6, 7 and 11). According to the literature, these reactions occur between 1.4% and 7% of all blood donations.30-34 Factor 2 shows high factor loadings for Items 1 (faintness), 4 (facial flush), 6 (difficulty hearing) and 9 (sweating). These symptoms are related to other factors that may affect the process of blood donation such as fear of needles or fear of seeing blood. These are dynamic mental processes that can be transformed into physical symptoms. It is known that strong emotions such as fear can cause neurovegetative symptoms related to alarming situations.35,36 The application of psychological strategies to minimize the impact of such fears, and consequently the appearance of unpleasant symptoms in the process of blood donation, are contemporary issues in transfusion medicine studies. Regarding Factor 3, factor loadings were observed with higher values for Items 8 (rapid or pounding heartbeat) and 10 (rapid or difficult breathing). These reactions are common in excited or anxious blood donors.37 Thus, considering the 11-item scale, Factors 1, 2 and 3 correspond to ‘vasovagal adverse reactions’, ‘fear’ and ‘anxiety/excitation’, respectively.
The 6- and 4-item scales have only one factor with an eigenvalue greater than 1. Thus, these scales are useful when the interest is to identify vasovagal symptoms related to blood donation alone, whereas the 11-item scale is useful if the aim is to assess not only vasovagal reactions, but also the donor’s psychological state directly related to the occurrence of such reactions.
The results shown in Table 3 suggest that since the 11-item scale addresses signs and symptoms related not only to the physiological response, but also the psychological reactions of the individual in relation to the drawing of blood, the nurse responsible for the donation is unable to have a complete notion about the sensations experienced by donors. Also, the 11-item scale described reactions in 91.7% of the cases of signs or symptoms recorded in the NARD, and when there was no record of a reaction, the 11-item scale identified that 76.5% of the donors reported no signs or symptoms during donation. This fact suggests that the NARD reported fewer reactions during blood donations than the BDRI, given that it is dependent on the direct observation of the nurse, whereas BDRI includes the subjective reactions of the donor. Therefore, it is noteworthy that one instrument does not replace the other as they have different purposes.
ConclusionIn conclusion, the findings suggest that the Portuguese version of the BDRI is a reliable and valid instrument to measure reactions towards blood donations. This research collaborates by providing a useful tool for research in transfusion medicine in Portuguese speaking countries.
Conflicts of interestThe authors declare no conflicts of interest.
AcknowledgementWe are grateful to FAEPA (HC-FMRP) and CNPq for financial support.
Indique o grau em que você experimentou as seguintes sensações ao doar sangue hoje circulando um número entre 0 (‘nem um pouco’) e 5 (‘extremadamente’).
| 0=nem um pouco | 3=forte | ||
| 1=leve | 4=muito forte | ||
| 2=moderado | 5=extremadamente |
| 1. Desmaio (como se estivesse prestes a desmaiar e ficar inconsciente) | 0 | 1 | 2 | 3 | 4 | 5 |
| 2. Tontura (sensação de desequilíbrio) | 0 | 1 | 2 | 3 | 4 | 5 |
| 3. Fraqueza | 0 | 1 | 2 | 3 | 4 | 5 |
| 4. Rubor na face (como se o rosto estivesse quente) | 0 | 1 | 2 | 3 | 4 | 5 |
| 5. Distúrbios visuais (tais como escurecimento da visão ou visão em túnel) | 0 | 1 | 2 | 3 | 4 | 5 |
| 6. Dificuldade em ouvir | 0 | 1 | 2 | 3 | 4 | 5 |
| 7. Vertigens (sensação que tudo está rodando) | 0 | 1 | 2 | 3 | 4 | 5 |
| 8. Batimento acelerado ou forte do coração | 0 | 1 | 2 | 3 | 4 | 5 |
| 9. Suor, sudorese, transpiração | 0 | 1 | 2 | 3 | 4 | 5 |
| 10. Respiração rápida ou dificuldade para respirar | 0 | 1 | 2 | 3 | 4 | 5 |
| 11. Náuseas (enjoos) ou dor de estômago | 0 | 1 | 2 | 3 | 4 | 5 |