Transfusion of ABO-compatible non-identical platelets (PTLs), fresh plasma (FP) and red blood cells (RBCs) has been associated with increased morbidity and mortality of recipients. Trauma victims are frequently exposed to ABO non-identical products, given the need for emergency transfusions. Our goal was to evaluate the impact of the transfusion of ABO non-identical blood products on the severity and all-cause 30-day mortality of trauma patients.
MethodsThis was a retrospective single-center cohort, which included trauma patients who received emergency transfusions in the first 24 h of hospitalization. Patients were divided in two groups according to the use of <3 or ≥3 ABO non-identical blood products. The patient severity, measured by the Acute Physiology and Chronic Health Evaluation (APACHEII) score at ICU admission, and the 30-day mortality were compared between groups.
ResultsTwo hundred and sixteen trauma patients were enrolled. Of these, 21.3% received ≥3 ABO non-identical blood products (RBCs, PLTs and FP or cryoprecipitate). The transfusion of ≥3 ABO non-identical blood products in the first 24 h of hospitalization was independently associated with a higher APACHEII score at ICU admission (OR = 3.28 and CI95% = 1.48–7.16). Transfusion of at least one unit of ABO non-identical PTLs was also associated with severity (OR = 10.89 and CI95% = 3.38–38.49). Transfusion of ABO non-identical blood products was not associated with a higher 30-day mortality in the studied cohort.
ConclusionThe transfusion of ABO non-identical blood products and, especially, of ABO non-identical PLTs may be associated with the greater severity of trauma patients at ICU admission. The transfusion of ABO non-identical blood products in the trauma setting is not without risks.
Blood transfusion is fundamental in the resuscitation of severe trauma patients.1 In the US, it is estimated that 10–15% of all red blood cell (RBC) units are used to treat trauma-injured victims.1 A subset of hemorrhaging patients may benefit from the early administration of RBCs, fresh plasma (FP) and platelets (PLTs), as part of a strategy named massive transfusion protocol, whose aim is to improve hemostasis and reduce the morbidity and mortality associated with severe trauma coagulopathy.2,3 The transfusion support for trauma victims commonly involves the initial selection of ABO compatible, but non-identical, blood products until the pre-transfusion tests are complete,4 given the need for emergency transfusions.
The deleterious effects associated with the transfusion of ABO non-identical blood products have been recently explored by some clinical studies, mostly focusing on surgical patients.5,6 The transfusion of ABO non-identical FP has already been associated with higher transfusion requirements, longer length of hospitalization and increased rates of acute respiratory distress syndrome (ARDS), sepsis and in-hospital mortality.6–8 Acute hemolytic reactions, poor transfusion increment and higher chances of post-transfusion reactions (febrile and allergic) have been described in association with the transfusion of ABO non-identical PLTs.9 Finally, a large study has demonstrated that transfusing group O RBC units to group A recipients significantly increases in-hospital mortality, irrespective of the transfusion indication.10
Despite the evidence pointing to the drawbacks of ABO non-identical transfusions, the selection of ABO non-identical blood products for transfusion is still commonplace, especially in emergency situations. Data referring to the deleterious impact of transfusing ABO non-identical blood products (RBCs, FP, PLT and cryoprecipitate (CRYO)) in the first 24 h of hospital care of trauma victims, as well as describing the patterns of the utilization of ABO non-identical blood products in the trauma setting, are yet incomplete.
Our main objective was to evaluate the impact of the transfusion of ABO non-identical blood products on the severity of trauma patients upon admission to the intensive care unit (ICU), using the Acute Physiology and Chronic Health Evaluation (APACHEII) score. As secondary goals, there are the evaluations of the association between the transfusion of ABO non-identical blood products on the 30-day all-cause mortality of the patients and the frequency and patterns of the utilization of ABO non-identical products (RBCs, FP, PLT and CRYO) in the trauma scenario.
MethodsStudy overviewThis was a retrospective single-center cohort study which included trauma patients admitted to the emergency department of a tertiary hospital from January 2016 to December 2018. The study was performed at the Hospital das Clínicas of the University of São Paulo and the blood products were issued by the Fundação Pró-Sangue São Paulo Hemocenter. Patients were included if they fulfilled the following inclusion criteria: 1) traumatic injury as the main cause of hospitalization; 2) age ≥18 years old; 3) transfusion of at least one blood product (RBC, FP, PLT or CRYO) in the first 24 h of in-hospital care, and; 4) admission to the intensive care unit (ICU) during the hospitalization. Patients were excluded for any of the following criteria: 1) dead on hospital arrival; 2) undetermined ABO group; 3) medical or transfusion records not available for consultation, and; 4) transfer to another hospital less than 12 h after admission. In this last situation, patients were most commonly transferred to private orthopedic centers.
In order to divide the studied cohort into two groups for analysis, the distribution of the number of ABO non-identical blood products transfused per patient was studied and the interquartile range was calculated. The option was to classify as ‘high’ the utilization of ABO non-identical blood products if the number of transfused units was superior to the upper (third) quartile. In this study, the upper quartile corresponded to ≥3 ABO non-identical blood products, which was used as threshold. The included patients were then divided into two groups according to the number of non-identical blood products (RBCs, FP, PLT or CRYO) they received in the first 24 h of hospitalization: 1) transfusion of <3 ABO non-identical blood products, and; 2) transfusion of ≥3 ABO non-identical blood products.
The impact of the use of ABO non-identical RBCs, FP, PLTs and CRYO on the target outcomes (mortality and high APACHE score) was also studied. Similarly to the explained before, the distribution of the use of each ABO non-identical blood product (RBC, FP, PLT or CRYO) per patient was evaluated and a high consumption of these products was considered, if in excess of the third (upper) quartile. This corresponded to ≥ 3 ABO non-identical RBCs, ≥1 ABO non-identical PLT and ≥1 non-identical FP. For statistical purposes, the number of ABO identical blood products transfused was considered as a potential confounder. The distribution of the transfusion of ABO identical blood products per patient was also evaluated and the threshold of 10 units was used as the reference, as it represented the upper quartile.
In the transfusion center studied, PLT units were either collected by apheresis or corresponded to a pool of 5 units of ABO and RhD identical random units. In this study, both the PLTs collected by apheresis and pool of random units were transfused. The CRYO units were not gathered in pools. None of the transfused cellular blood products were leukodepleted, with the exception of the PLT units.
Transfusion was considered as ABO non-identical in the situation in which the ABO type of the blood product differed from that presented by the patient. The volume of plasma per unit of FP and PLTs is approximately 200 and 250–350 mL, respectively, and, in the case of an ABO non-identical transfusion involving FP or PLTs, this would be the transfused volume of plasma containing incompatible isohemagglutinins. In the case of RBC units, the volume of remaining plasma is less than 50 mL. Thus, transfusions of ABO non-identical RBC units are associated with a significantly lower infusion of incompatible plasma, in comparison to FP and PLTs.
The study was approved by the local ethics committee and was conducted following the Helsinki principles.
Studied variablesDemographic, clinical, transfusion and laboratorial data of the included patients were obtained from the hospital and blood bank electronic files.
Results of blood gas analysis (pH, pO2, pCO2, BE, HCO3 and SatO2), hemoglobin / hematocrit and lactate collected upon the arrival of patients at the emergency department were retrieved from electronic medical records. Considering that the severity of the trauma patients was not accessed by any scores upon arrival, the hemoglobin and lactate levels upon hospital admission, which reflected the severity of the victims, were obtained from the laboratory records and used in the statistical analysis to avoid biased comparisons. The mechanisms of trauma and the need for emergency surgery were also analyzed.
The full list of all blood products (RBCs, FP, PLTs and CRYO) issued and transfused to the patients during the first 24 h of hospitalization and after 24 h of hospitalization was compiled. The ABO patient and transfused blood products groups were determined using the conventional tube method and the results were used to classify the transfusions as ABO identical or non-identical.
The APACHEII score was calculated for all participants upon arrival at the ICU, following the instructions described elsewhere.11 The variables included in the APACHEII represented the worst physiological variables within the first 24 h of ICU admission and were represented by: age, Glasgow Coma Score (GCS), temperature, mean arterial pressure, heart rate, respiratory rate, FiO2, PaO2, arterial pH, sodium, potassium, creatinine, acute renal failure, hematocrit, leukocyte count and presence of organ system insufficiency or immunodeficiency. The APACHEII score was chosen to evaluate the severity of the patients because this was the prognostic tool applied at our service from 2016 to 2018.
Mortality outcome (dead or alive) 30 days after hospital admission was retrieved from the medical files. In case of missing data, the family was contacted via telephone to inform this endpoint.
Statistical analysisContinuous variables were expressed in terms of median / interquartile rank and compared between the two groups using the Mann-Whitney test. Categorical variables were computed by direct counting and the proportion of cases was compared between groups using the Chi-square or Fisher tests, as appropriate. All tests were two-tailed.
As multivariable analysis, we conducted logistic regression to estimate the risks of a high APACHEII score (≥20 points) upon admission to the ICU and death at 30 days. Clinical and laboratorial variables associated with the dependent variables (APACHEII ≥ 20 and 30-day mortality) with p ≤ 0.2 in univariate analysis were included in the models.
A p-value less than 0.05 was considered significant for all tests. All statistical analyses were performed in R software.
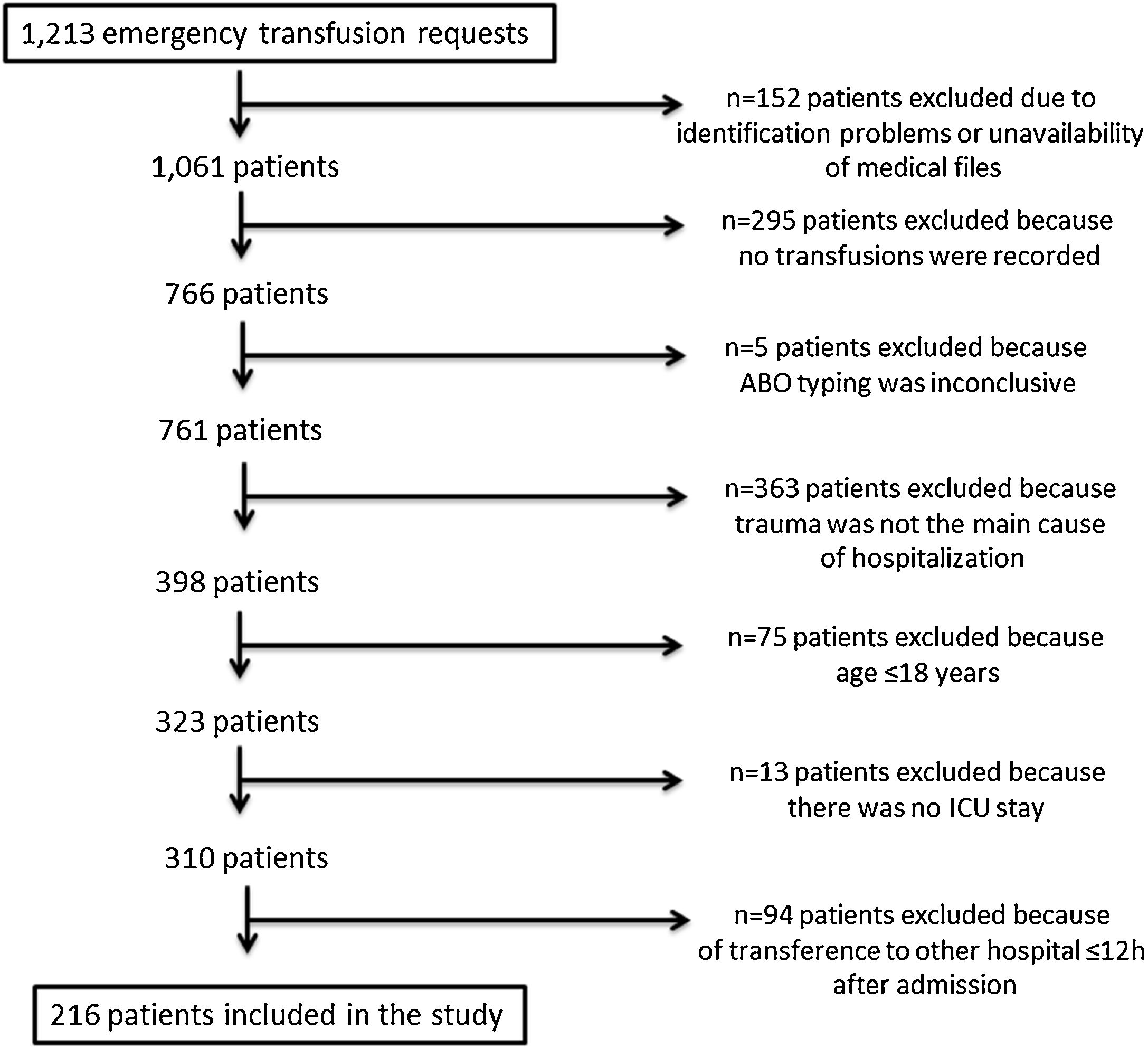
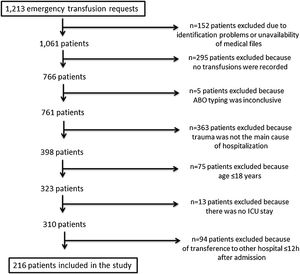
ResultsPatient recruitmentA full list of all requests for emergency transfusions was produced from the blood bank electronic system. Two hundred and sixteen patients fulfilled the inclusion criteria and were enrolled in this study. Figure 1 displays the patient recruitment process.
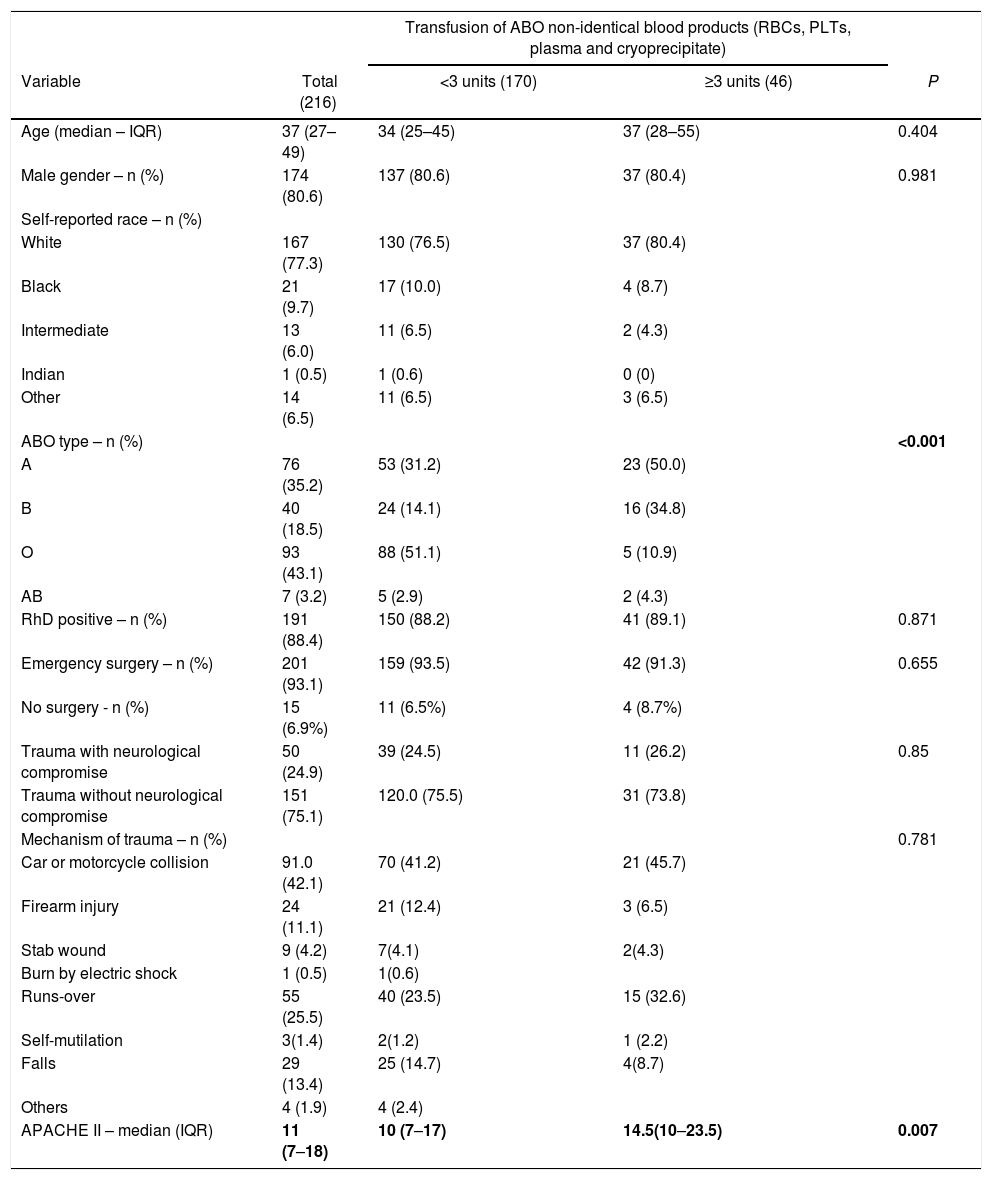
Demographics of the included patientsThe demographical characterization of the patients taking part in the study is described in Table1. The mean time between hospital and ICU admission was 16.2 ± 6.49 h. In general, patients were young (median age = 37 and interquartile range (IQR) = 27–49) and mostly male (80.6%). The distribution of ABO groups reflected that of the Brazilian population, with 43.1% of the patients typing as O and 35.2% of the patients typing as A. As expected, in the group of patients that received less than 3 units of ABO non-identical blood products, most patients were of group O (51.1%), whereas patients of group A represented the majority in the group that received 3 or more ABO non-identical blood products (50%).
Demographics of the studied cohort of patients.
| Transfusion of ABO non-identical blood products (RBCs, PLTs, plasma and cryoprecipitate) | ||||
|---|---|---|---|---|
| Variable | Total (216) | <3 units (170) | ≥3 units (46) | P |
| Age (median – IQR) | 37 (27–49) | 34 (25–45) | 37 (28–55) | 0.404 |
| Male gender – n (%) | 174 (80.6) | 137 (80.6) | 37 (80.4) | 0.981 |
| Self-reported race – n (%) | ||||
| White | 167 (77.3) | 130 (76.5) | 37 (80.4) | |
| Black | 21 (9.7) | 17 (10.0) | 4 (8.7) | |
| Intermediate | 13 (6.0) | 11 (6.5) | 2 (4.3) | |
| Indian | 1 (0.5) | 1 (0.6) | 0 (0) | |
| Other | 14 (6.5) | 11 (6.5) | 3 (6.5) | |
| ABO type – n (%) | <0.001 | |||
| A | 76 (35.2) | 53 (31.2) | 23 (50.0) | |
| B | 40 (18.5) | 24 (14.1) | 16 (34.8) | |
| O | 93 (43.1) | 88 (51.1) | 5 (10.9) | |
| AB | 7 (3.2) | 5 (2.9) | 2 (4.3) | |
| RhD positive – n (%) | 191 (88.4) | 150 (88.2) | 41 (89.1) | 0.871 |
| Emergency surgery – n (%) | 201 (93.1) | 159 (93.5) | 42 (91.3) | 0.655 |
| No surgery - n (%) | 15 (6.9%) | 11 (6.5%) | 4 (8.7%) | |
| Trauma with neurological compromise | 50 (24.9) | 39 (24.5) | 11 (26.2) | 0.85 |
| Trauma without neurological compromise | 151 (75.1) | 120.0 (75.5) | 31 (73.8) | |
| Mechanism of trauma – n (%) | 0.781 | |||
| Car or motorcycle collision | 91.0 (42.1) | 70 (41.2) | 21 (45.7) | |
| Firearm injury | 24 (11.1) | 21 (12.4) | 3 (6.5) | |
| Stab wound | 9 (4.2) | 7(4.1) | 2(4.3) | |
| Burn by electric shock | 1 (0.5) | 1(0.6) | ||
| Runs-over | 55 (25.5) | 40 (23.5) | 15 (32.6) | |
| Self-mutilation | 3(1.4) | 2(1.2) | 1 (2.2) | |
| Falls | 29 (13.4) | 25 (14.7) | 4(8.7) | |
| Others | 4 (1.9) | 4 (2.4) | ||
| APACHE II – median (IQR) | 11 (7–18) | 10 (7–17) | 14.5(10–23.5) | 0.007 |
Most patients underwent emergency surgery (93.1%). A small fraction of patients were not subjected to surgery (n = 15, 6.9%) and represented cases of extreme severity. The most important mechanisms of trauma were car or motorcycle collision (42.1%), followed by run-overs by motor vehicles (25.5%).
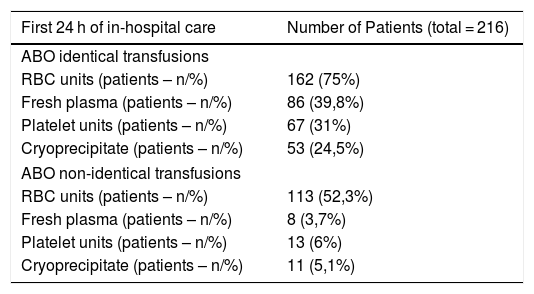
Distribution of ABO identical and ABO non-identical transfusionsIn the studied cohort, the median number of ABO identical blood products transfused in the first 24 h of care was 3 (IQR 1–10) and the median number of ABO non-identical blood products transfused was 2 (IQR 0–2). One hundred and seventy patients received less than three ABO non-identical blood products (RBCs, PLTs, FP or CRYO), while 46 were transfused with three or more ABO non-identical units (Table 2).
Distribution of ABO identical and non-identical transfusions in the studied cohort of trauma patients.
| First 24 h of in-hospital care | Number of Patients (total = 216) |
|---|---|
| ABO identical transfusions | |
| RBC units (patients – n/%) | 162 (75%) |
| Fresh plasma (patients – n/%) | 86 (39,8%) |
| Platelet units (patients – n/%) | 67 (31%) |
| Cryoprecipitate (patients – n/%) | 53 (24,5%) |
| ABO non-identical transfusions | |
| RBC units (patients – n/%) | 113 (52,3%) |
| Fresh plasma (patients – n/%) | 8 (3,7%) |
| Platelet units (patients – n/%) | 13 (6%) |
| Cryoprecipitate (patients – n/%) | 11 (5,1%) |
Referring to the transfusion of ABO non-identical blood products, 52.3% of the overall cohort received ABO non-identical RBCs, 6% received ABO non-identical PLTs, 5.1% received ABO non-identical CRYO and 3.7% received ABO non-identical FP (Table 2).
In the group of patients receiving three or more ABO non-identical blood products in the first 24 h of hospitalization, transfusion of ABO non-identical RBCs represented the most common cause of ABO non-identical transfusion (89.1% of the individuals), followed by ABO non-identical PLTs (28.3%), ABO non-identical CRYO (19.6%) and ABO non-identical FP (13%) (Table 2).
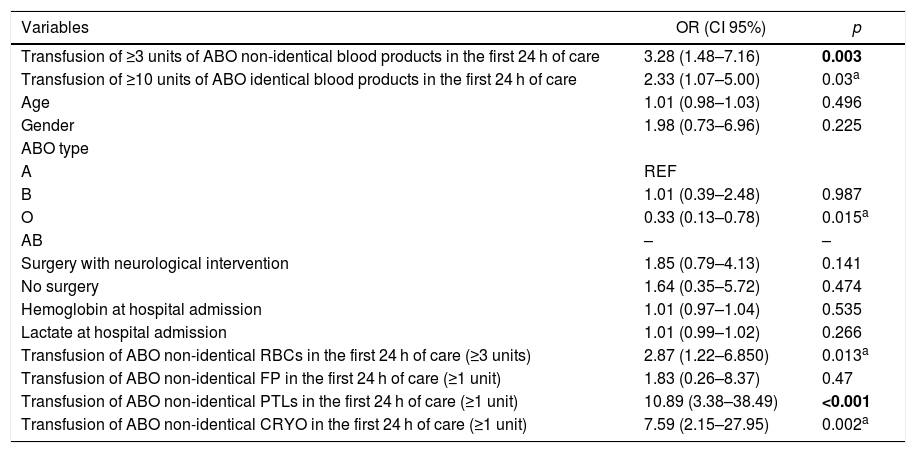
Impact of the transfusion of ABO non-identical blood products on the severity of the studied trauma patientsThe APACHEII score calculated at ICU admission was significantly higher in the group of patients receiving ≥3 units of ABO non-identical blood products (median 14.5, IQR 10–23.5), in comparison to that of patients receiving less than 3 units of ABO non-identical transfusions (median = 10 and IQR = 7–17) (p = 0.007, Table 1). The following variables were significantly associated with the higher severity of the studied patients (APACHEII ≥ 20) upon admission to the ICU in the univariate analysis: transfusion of 3 or more units of ABO non-identical blood products (p = 0.003), transfusion of 10 or more units of ABO identical blood products (p = 0.03), ABO group O (p = 0.015), transfusion of 3 or more units of ABO non-identical RBC units (p = 0.013), transfusion of 1 or more units of ABO-non identical PLT units (p < 0.001) and transfusion of 1 or more units of ABO non-identical CRYO (p = 0.002). The age and gender of the victim, type of surgery, hemoglobin and lactate upon admission to the emergency room and transfusion of 1 or more units of ABO non-identical FP were not significantly associated with a higher APACHEII score upon admission to the ICU (Table 3).
Analysis of the variables associated with higher APACHE II score (≥20) of trauma victims upon admisstion to the ICU.
| Variables | OR (CI 95%) | p |
|---|---|---|
| Transfusion of ≥3 units of ABO non-identical blood products in the first 24 h of care | 3.28 (1.48–7.16) | 0.003 |
| Transfusion of ≥10 units of ABO identical blood products in the first 24 h of care | 2.33 (1.07–5.00) | 0.03a |
| Age | 1.01 (0.98–1.03) | 0.496 |
| Gender | 1.98 (0.73–6.96) | 0.225 |
| ABO type | ||
| A | REF | |
| B | 1.01 (0.39–2.48) | 0.987 |
| O | 0.33 (0.13–0.78) | 0.015a |
| AB | – | – |
| Surgery with neurological intervention | 1.85 (0.79–4.13) | 0.141 |
| No surgery | 1.64 (0.35–5.72) | 0.474 |
| Hemoglobin at hospital admission | 1.01 (0.97–1.04) | 0.535 |
| Lactate at hospital admission | 1.01 (0.99–1.02) | 0.266 |
| Transfusion of ABO non-identical RBCs in the first 24 h of care (≥3 units) | 2.87 (1.22–6.850) | 0.013a |
| Transfusion of ABO non-identical FP in the first 24 h of care (≥1 unit) | 1.83 (0.26–8.37) | 0.47 |
| Transfusion of ABO non-identical PTLs in the first 24 h of care (≥1 unit) | 10.89 (3.38–38.49) | <0.001 |
| Transfusion of ABO non-identical CRYO in the first 24 h of care (≥1 unit) | 7.59 (2.15–27.95) | 0.002a |
In bold: statistical relevant association in the multivariate analysis. The multivariate model included the following variables: transfusion of more than 10 ABO identical blood products in the first 24 h. age. ABO type. transfusion of ABO non-identical RBCs. PTLs and CRYO in the fisrt 24 h.
Two models of multivariate analysis were elaborated. In the first, transfusion of 3 or more ABO non-identical blood products was evaluated, adjusting for the total number of ABO identical transfusions (≥10 units) and age. The positive association between transfusion of ABO non-identical blood products and a higher APACHEII score was statistically relevant (p = 0.011; OR = 2.88; 95%CI = 1.25–6.53) (Table 3). In the second model, the effect of the transfusion of ABO non-identical RBCs, PLT, CRYO and FP were individually studied, adjusting for the number of identical transfusions (≥10 units) and age. Transfusion of one or more ABO non-identical PLT units was independently associated with a higher APACHE II score (p = 0.026; OR = 5.22; 95%CI = 1.23–23.82). Transfusion of ABO non-identical RBCs, FP and CRYO were not significantly associated with the selected outcome (Table 3).
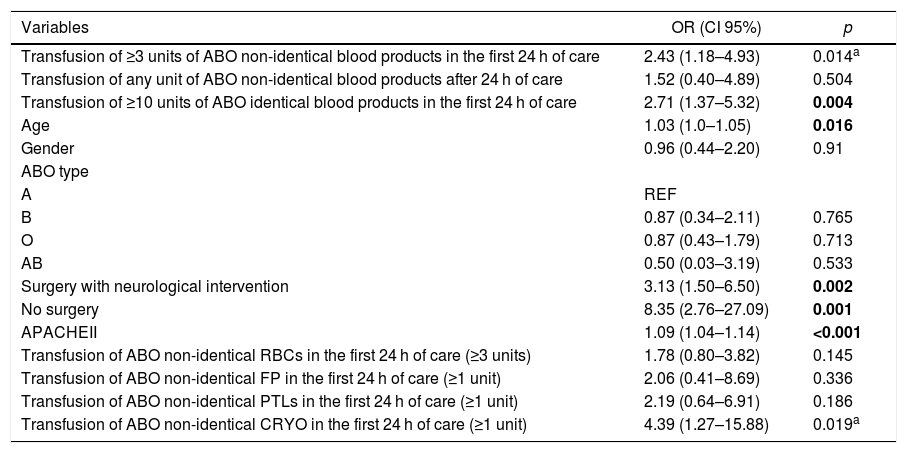
Mortality analysisThere were 50 deaths in the studied cohort in a 30-day follow-up, resulting in a mortality rate of 23.1% (CI 95% = 17.7%–29.3%). Data referring to the cause of death was retrieved in 25 cases: sepsis (n = 18), respiratory distress (n = 4) and acute renal failure (n = 3). In the univariate analysis, the following variables were positively associated with increased mortality: transfusion of ≥3 units of ABO non-identical blood products in the first 24 h of hospitalization, transfusion of ≥10 units of ABO identical blood products in the first 24 h of hospitalization, age, surgery with neurological intervention, no surgery, APACHEII score and transfusion of ≥1 unit of ABO non-identical cryoprecipitate (Table 4). The transfusion of ABO non-identical products after 24 h of hospitalization, gender, ABO type, transfusion of ABO non-identical RBCs (≥3 units), plasma (≥1 unit) and PLTs (≥1 unit) were not significantly associated with the studied endpoint (Table 4).
Analysis of the variables associated with all-cause 30-day mortality of the studied cohort.
| Variables | OR (CI 95%) | p |
|---|---|---|
| Transfusion of ≥3 units of ABO non-identical blood products in the first 24 h of care | 2.43 (1.18–4.93) | 0.014a |
| Transfusion of any unit of ABO non-identical blood products after 24 h of care | 1.52 (0.40–4.89) | 0.504 |
| Transfusion of ≥10 units of ABO identical blood products in the first 24 h of care | 2.71 (1.37–5.32) | 0.004 |
| Age | 1.03 (1.0–1.05) | 0.016 |
| Gender | 0.96 (0.44–2.20) | 0.91 |
| ABO type | ||
| A | REF | |
| B | 0.87 (0.34–2.11) | 0.765 |
| O | 0.87 (0.43–1.79) | 0.713 |
| AB | 0.50 (0.03–3.19) | 0.533 |
| Surgery with neurological intervention | 3.13 (1.50–6.50) | 0.002 |
| No surgery | 8.35 (2.76–27.09) | 0.001 |
| APACHEII | 1.09 (1.04–1.14) | <0.001 |
| Transfusion of ABO non-identical RBCs in the first 24 h of care (≥3 units) | 1.78 (0.80–3.82) | 0.145 |
| Transfusion of ABO non-identical FP in the first 24 h of care (≥1 unit) | 2.06 (0.41–8.69) | 0.336 |
| Transfusion of ABO non-identical PTLs in the first 24 h of care (≥1 unit) | 2.19 (0.64–6.91) | 0.186 |
| Transfusion of ABO non-identical CRYO in the first 24 h of care (≥1 unit) | 4.39 (1.27–15.88) | 0.019a |
In bold: statistical relevant association in the multivariate analysis. The following variables were included in the multivariate model: transfusion of more than 3 units of ABO non-identical blood products. transfusion of more than 10 ABO compatible units in the firts 24 h. age. surgery with neurological intervention. no surgery. APACHEII. transfusion of ABO non-identical RBCs, PTLs and CRYO.
In the multivariate model, age (p = 0.021), surgery with neurological intervention (p = 0.006), no surgery (p < 0.001), APACHEII score (p = 0.033) and transfusion of ≥10 units of blood products (p = 0.013) were significantly associated with mortality, while transfusion of ≥3 units of ABO non-identical blood products in the first 24 h of hospitalization and transfusion of ≥1 unit of ABO non-identical cryoprecipitate did not reach statistical significance.
DiscussionThis study evaluated the impact of ABO non-identical transfusions on the severity and 30 day- mortality of a cohort of trauma patients undergoing emergency transfusions. It was demonstrated that: 1) more than half of the trauma patients received at least one unit of ABO non-identical RBCs in the first 24 h of hospitalization and more than 20% were transfused with three or more ABO non-identical blood products in the same period; 2) the transfusion of three or more ABO non-identical blood products in the first 24 h of hospitalization was independently associated with an increase in the APACHEII score upon admission to the ICU; 3) the transfusion of at least one unit of ABO non-identical PLTs in the first 24 h of hospitalization was also associated with an increased APACHEII score at ICU admission, and; 4) in the studied cohort, transfusion of three or more units of ABO non-identical blood products was not independently associated with an increase in the all-cause 30-day mortality rates. The same applied to the transfusion of each ABO non-identical blood product independently (RBCs, PLTs, FP and CRYO).
The present study provided an overview of the patterns of the utilization of ABO non-identical blood products to transfuse trauma patients. In general, RBCs represented the product most commonly associated with situations of ABO non-identical transfusions, as approximately half of the patients received at least one ABO non-identical unit. This probably reflects the fact that the victims were transfused with ABO non-identical blood products until the ABO type was determined and, considering that RBC units were prescribed first, the rates of transfusion of ABO non-identical RBC units were higher, in comparison to other blood products. In contrast, less than one third of the patients receiving ≥3 ABO non-identical blood products were transfused with ABO non-identical PLTs or FP. Furthermore, in the studied cohort, the median number of ABO identical blood transfusions was not high, indicating that non-severe patients were included and, consequently, the exposure to PLTs, FP and CRYO was lower. Even so, the percentage of patients transfused with three or more ABO non-identical blood products was not low (21.3%), nor was the percentage of patients receiving ABO non-identical FP and PLTs (3.7% and 6%, respectively). This suggests that the prompt determination of the patient ABO group and the selection of ABO identical products for transfusion occurred at a lower than expected frequency.
Our results have demonstrated an independent association between the transfusion of at least one unit of ABO non-identical PLTs and the greater severity of trauma patients upon admission to the ICU. This data is novel in the literature, as the study specifically focused on the patient severity after the initial care in the emergency department, and reinforces previous evidence of the drawbacks associated with the transfusion of ABO non-identical blood products of high plasma content (PLTs and FP) to both surgical and trauma patients. In this regard, previous reports have associated the exposure to ABO non-identical PLTs or FP with increased RBC transfusion needs and the length of hospitalization in surgical patients.6 In the trauma scenario, transfusion of ABO non-identical FP has already been significantly associated with higher overall complication rates (acute respiratory distress syndrome (ARDS) and sepsis) of the victims.7
There are some theories to explain the deleterious effect of the transfusion of ABO non-identical PLTs, evidenced by the present and previous studies. First, circulating immune complexes (CIC) that form when incompatible ABO antibodies or antigens are transfused may cause the suppression of cellular immunity, with inappropriate activation of inflammatory pathways, contributing to the occurrence of clinical adverse events.5 Moreover, the transfusion of ABO non-identical blood products of high plasma content (PLTs and FP) may lead to PLT and/or endothelial dysfunction due to the action of ABO antibodies or CICs that interfere with hemostasis, exacerbating bleeding, hemolysis and increasing the RBC transfusion needs.6 Lastly, the ABO antibodies present in the PLTs may exert direct damage to susceptible organs by causing microvasculature damage. This mechanism has originally been described among patients undergoing allogenic stem cell transplantation, who experienced an increase in the regimen-related toxicity with the transfusion of ABO non-identical PLTs,12 but can potentially be extrapolated to the trauma scenario, considering the systemic inflammation and organ damage of the victims.
The transfusion of ABO non-identical RBCs, PLTs, FP and CRYO was not associated with an increased 30-day mortality in the studied cohort. This association has been previously described for both ABO non-identical FP and RBCs. For FP, Shanwell et al. have detected an increase in mortality associated with exposure to ABO non-identical products, but confined to the patients receiving more than five units.8 For RBCs, Pai et al. described an increased risk of in hospital death due to the transfusion of type O RBC units to group A recipients in a large retrospective cohort including more than 18,000 patients.10 One reason why these results could not be reproduced in our cohort is the number of included patients, as only a very small number of the included participants received a large amount of ABO non-identical blood products. Our results, however, show an increased APACHEII score among the patients receiving more than two ABO non-identical blood products or at least one unit of ABO non-identical PLTs. The APACHEII score is an important mortality predictor tool, which accurately estimates the ICU mortality. As such, differences in mortality could potentially have been detected if the number of included patients had been larger.
Our data sheds light on the deleterious effects of the transfusion of ABO non-identical PLT units to trauma patients. Even though the disadvantages of the transfusion of ABO non-identical PLTs are well documented, major or minor incompatibilities with the transfusion of PLTs with ABO are not uncommon, mainly due to the risks of product outdating and the complex logistics associated with the sole transfusion of ABO identical products.9,13 In the trauma setting, the transfusion of ABO identical PLTs would be possible with the rapid determination of the victim’s ABO group, associated with a considerable amount of PLT units available to meet the transfusion needs of non-group O recipients. It has already been demonstrated that implementing a policy of providing ABO identical-only PLT transfusions to all patients reduces the rates of transfusion reactions (febrile and allergic), as well as the development of post-transfusion RBC antibodies, and, as a consequence, is recommended. The slight increase in product outdating associated with this protocol would be compensated by a large decrease in the rate of ABO non-identical transfusions,14 what may be clinically advantageous.
This study has some limitations. The first one refers to the number of included patients, which was not large, considering this was a single-center study. The benefits of having only one center recruiting patients is that the protocols of care and transfusion applied throughout the study were uniform. Another limitation was that no severity scores were calculated for the patients upon admission to the hospital. As such, hemoglobin and lactate upon arrival were used as parameters of the patient severity for statistical purposes. Finally, this was a retrospective study and, as a consequence, the study design was not ideal for the risk assessment. The conclusions derived from the study are valid, but need to be validated by large prospective trials.
In conclusion, the transfusion of ABO non-identical blood products may increase the severity of trauma patients upon admission to the ICU. Future studies are needed to determine if the restricted transfusion of ABO non-identical blood products in the trauma setting is clinically advantageous.
Conflicts of interestThe author declares no conflicts of interest.
This study was funded by the Fundação Pró-Sangue Hemocentro de São Paulo (no grant number).