Drug interference in serological assays is a well-known phenomenon. Therapeutic monoclonal antibodies (MoAbs) have been successful in treating a variety of malignancies. Some of these MoAbs interfere in day-to-day laboratory tests. Recently, a novel targeted immunotherapy, daratumumab (MoAb against CD38 antigen) was approved for treating advanced stage multiple myeloma (MM).1 Herein, we describe the interference of daratumumab in the red blood cell (RBC) compatibility testing of a patient with refractory relapsed MM.
Case descriptionA 72-year-old gentleman previously diagnosed with immunoglobulin (Ig)Aλ MM presented with complaints of pain in both the hip joints for three weeks. Hematological parameters at the time of admission were: hemoglobin 6.8g/dL, platelet count: 95×109/L; total leukocyte count: 2.7×103/μL. Magnetic resonance imaging (MRI) showed spinal cord compression at L4-L5 level. On the second week of admission, we received a request for one RBC unit. His blood grouping was B RhD positive. Initially, one RBC unit was incompatible in the anti-human globulin (AHG) phase using a LISS/Coombs gel card (Biorad, Cressier, Switzerland). Immediate spin (IS) crossmatch using the tube technique was compatible. A direct antiglobulin test (DAT) of the patient was negative for IgG and C3d using monospecific Coombs gel card (Biorad, Cressier, Switzerland). Subsequently, we performed an indirect antiglobulin test (IAT) using LISS/Coombs gel card with commercial three cell panel, DiaCell I-II-III (Biorad, Cressier, Switzerland) which was panreactive (2+) while the auto control was negative. RBC antibody identification was panreactive (2+) using the ID-Diapanel (Biorad, Cressier, Switzerland). We suspected multiple alloantibodies or an antibody to a high-prevalence antigen. Extended phenotyping could not be done as the previous transfusion was within three months. RBC antibody identification was repeated using a papain treated 11-cell panel (single stage assay). However, no variation in the grade of agglutination was observed. Acid elution was performed using the commercial kit (Diacidel, Biorad, Cressier, Switzerland) and the IAT of the eluate was negative. We further performed AHG crossmatch with 18 group specific RBC units and all were incompatible. Previously, the patient had been transfused on different occasions at our center with five group specific packed RBCs (AHG compatible) due to anemia. We reviewed his compatibility profile prior to the first transfusion. His blood group was B RhD positive, while his DAT and IAT were negative. Retrospective examination of his treatment records revealed that the patient had received one cycle of daratumumab (dose: 16mg/kg – 1200mg) three days before the crossmatching was requested. Since the RBC units were AHG compatible prior to the initiation of daratumumab, we suspected that daratumumab interfered in pretransfusion testing.
Dithiothreitol (DTT) treatment of the reagent RBCs has been shown to eliminate the effects of daratumumab. So, we attempted crossmatching with DTT treated donor RBCs as described by Chapuy et al.2 DTT (0.2M) was prepared by dissolving 1g of DTT in 32mL of phosphate buffered saline (PBS) at pH 8.0. Two K-negative RBC units were taken for crossmatching. K+ and E+ RBCs were used as controls. Donor cells and control cells were washed with PBS (pH 7.3) four times. A quantity of 400μL DTT at pH 8.0 was added to 100μL (5% suspension) of RBCs. The mixture was incubated at 37°C for 30min. Following incubation, the cells were washed with PBS (pH 7.3) four times. A 0.8% suspension was prepared with PBS (pH 7.3) and the crossmatch was repeated on a LISS/Coombs gel card. After DTT treatment, the two RBC units were AHG-compatible. IAT was negative with DTT-treated reagent RBCs. The patient was transfused with one compatible RBC unit and no transfusion reaction was observed. Post-transfusion, the patient's hemoglobin level improved to 7.6g/dL.
DiscussionDaratumumab is the first-in-class human IgG1κ anti-CD38 MoAb approved by the FDA in 2015 for the treatment of patients with MM who are double refractory to immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs). CD38 is a transmembrane glycoprotein which is involved in cell adhesion and signal transduction in a variety of cells. It plays a key role in intracellular calcium mobilization through its enzymatic activity. It is normally expressed at low levels on precursor and activated T and B cells, myeloid cells, natural killer cells, RBCs, platelets and plasma cells. It is strongly expressed on myeloma cells, a fact which underlies the treatment of MM with targeted immunotherapy.1 False positive IAT results were first observed in patients receiving daratumumab during phase I and II clinical trials.3 However, the drug does not affect the patient's ABO blood grouping and immediate spin crossmatch. It binds to endogenous CD38 on RBCs. Such sensitized RBCs agglutinate in the Coombs phase resulting in panreactivity in vitro (DAT, antibody screening/identification, crossmatching).4
Routine serological methods are ineffective in circumventing the interference in compatibility testing, thereby resulting in an unexpected delay in finding suitable blood for these patients. Also, it may mask the underlying alloantibodies, mimicking a high titer antibody or an antibody to a high-prevalence antigen as described in our case.5 Anti-CD38 can bind to the patient's own RBCs causing positive DAT. In our case, the DAT was negative which probably was due to clearance of drug-coated RBCs in the spleen by Fc-receptor mediated uptake. Daratumumab infusion has been related to clinically insignificant anemia.6 So far, after the drug's infusion, no transfusion-related hemolysis has been observed in several clinical trials held worldwide.5 After starting daratumumab in our patient, drug related anemia was not observed.
Several strategies have been proposed to eliminate the drug's interference. Provision of blood for such patients is feasible by 1. Thiol treated RBCs for pretransfusion testing; 2. serologic phenotyping of patients and antigen-matched transfusions; 3. Genotyping and antigen-matched transfusions; 4. antibody screening with cord blood 5. neutralizing the antibody with soluble CD38 or anti-daratumumab and 6. a combination of these techniques.7
Genotyping is more accurate and comprehensive in providing extended antigen profile of RBCs than serologic phenotyping. It can be carried out in patients even after the initiation of daratumumab. This method is expensive, not readily available and challenging for many centers for want of an antigen-matched inventory. As per previous reports, many centers perhaps prefer the DTT-based technique thanks to its easy availability in blood banks. CD38 has six disulfide bonds in its extracellular domain which are disrupted by DTT and other reagents (2-mercaptoethanol/2-aminoethylisothiouronium). Very few centers have reported the use of the other thiol reagents.2 Our center is a tertiary care hospital and a transplant center (bone marrow and solid-organ transplant). However, we lack facilities for antigen genotyping. We routinely use DTT for ABO antibody titers. Hence, we preferred DTT-based pretransfusion testing to negate the drug's interference. Since DTT denatures Kell antigens, K-negative RBC units should be provided for such patients unless the patient is K-positive. However, this is not without disadvantages. Firstly, it is time-consuming (average time taken to resolve the interference in a patient with no antibody and with one alloantibody was 2h and 4h, respectively). Secondly, underlying antibodies against K, DO, IN, JMH, KN, LW antigens would not be detected, as these antigens are destroyed by DTT. Nevertheless, this situation is very rare.2
Anani et al., evaluated and compared the additional costs involved in three different strategies (thiol-based antibody investigation, phenotyping or genotyping and antigen-matched transfusions) for initial and subsequent transfusions using breakeven analysis from various immunohematology reference laboratories. The following observations were made: The most commonly ordered test was thiol-based antibody investigation. Genotyping was preferred to phenotyping for antigen matching as it is precise (detects silent mutations) and relatively cheaper. Thiol-based antibody investigation appeared to be the least expensive method for the initial transfusion (single event). However, for multiple transfusions, genotyping and antigen-matched transfusions would be cost-effective and time-saving when compared to thiol-based repeat antibody investigations. Four and five antigen-matched transfusions by genotyping equalled the cost of thiol-based antibody investigations within five and 21 transfusion events, respectively. In contrast, for more than six antigen-matched transfusions, thiol-based antibody investigation was economical.7
Other approaches have limited role in many centers owing to their limited availability, high-cost and lack of standardization. The drug's effect is thought to persist for six months after initiation.1 In this patient, for future transfusions, antibody screening would be repeated with DTT-treated cells. If the antibody screening is negative, the patient will be provided K−, ABO/D compatible RBCs by IAT crossmatch using DTT treated donor RBCs. If the antibody screening is positive, DTT-antibody identification will be performed. Antigen negative units would be provided by IAT crossmatch using DTT treated donor RBCs.8 Also, we have provided the patient with an information card indicating his treatment with daratumumab.
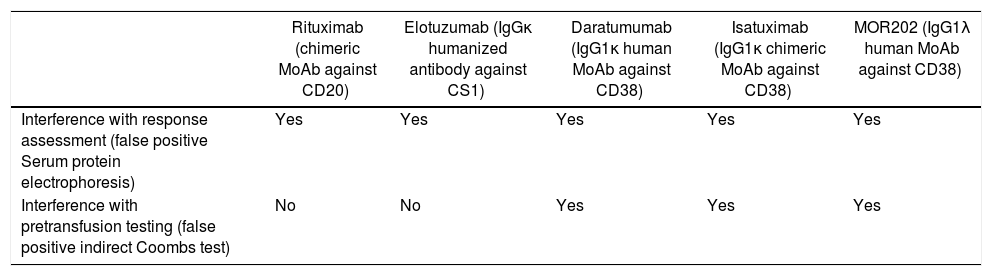
Other anti-CD38 MoAbs currently under investigation such as chimeric IgGκ MoAb isatuximab (SAR650984) and IgG1λ MoAb MOR202 are also expected to cause false positive IAT results due to their class effect (Table 1).1 Anti-CD44 MoAbs are also thought to interfere with pretransfusion testing.9 Daratumumab, similar to rituximab, is also implicated in false positive results in serum protein electrophoresis and immunofixation electrophoresis, thereby affecting the monitoring of complete response in patients with IgG1κ myeloma. Flow cytometric assessment of normal and neoplastic plasma cells is also hampered following the initiation of daratumumab. The anti-CD38 drugs are currently being evaluated for their efficacy in other hematological malignancies (non-Hodgkin lymphoma, chronic lymphocytic leukemia).10 This unique clinical laboratory problem is a must know entity for clinicians and transfusion medicine specialists. It is also emphasized that communication to the blood bank about the initiation of daratumumab for MM patients is crucial to avoid unnecessary delay in the release of compatible units.
Monoclonal antibodies and their interference in laboratory investigations.
| Rituximab (chimeric MoAb against CD20) | Elotuzumab (IgGκ humanized antibody against CS1) | Daratumumab (IgG1κ human MoAb against CD38) | Isatuximab (IgG1κ chimeric MoAb against CD38) | MOR202 (IgG1λ human MoAb against CD38) | |
|---|---|---|---|---|---|
| Interference with response assessment (false positive Serum protein electrophoresis) | Yes | Yes | Yes | Yes | Yes |
| Interference with pretransfusion testing (false positive indirect Coombs test) | No | No | Yes | Yes | Yes |
Daratumumab and other anti-CD38 MoAbs are emerging as a promising targeted approach for patients with refractory relapsed MM. However, they are not without limitations. These drugs interfere with compatibility testing thereby leading to misinterpretations of test results and delay in blood transfusions. Time-consuming special techniques are needed to nullify the drug's interference. Every center should recognize its mode of testing based on the accessibility of reagents, availability of manpower and expertise, antigen-matched blood and its inventory and turn around time. Of note, the DTT-based method has been accepted worldwide. Communication to the Transfusion Medicine Department regarding treatment with such drugs is mandatory to ensure safe transfusions in these patients. Knowledge related to these inadvertent adverse effects is essential for optimizing transfusion requirements in these patients.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Ms. Manimegalai, Lab Technologist for carrying out the technical aspects involved in this study.