Febrile neutropenia is an important cause of mortality and morbidity in hematology–oncology patients undergoing chemotherapy. The management of febrile neutropenia is typically algorithm-driven. The aim of this study was to assess the results of a standardized protocol for the treatment of febrile neutropenia.
MethodsA retrospective cohort study (2011–2012) was conducted of patients with high-risk neutropenia in a hematology–oncology service.
ResultsForty-four episodes of 17 patients with a median age of 48 years (range: 18–78 years) were included. The incidence of febrile neutropenia was 61.4%. The presence of febrile neutropenia was associated with both the duration and severity of neutropenia. Microbiological agents were isolated from different sources in 59.3% of the episodes with bacteremia isolated from blood being the most prevalent (81.3%). Multiple drug-resistant gram-negative bacilli were isolated in 62.5% of all microbiologically documented infections. Treatment of 63% of the episodes in which the initial treatment was piperacillin/tazobactam needed to be escalated to meropenem. The mortality rate due to febrile neutropenia episodes was 18.5%.
ConclusionThe high rate of gram-negative bacilli resistant to piperacillin/tazobactam (front-line antibiotics in our protocol) and the early need to escalate to carbapenems raises the question as to whether it is necessary to change the current protocol.
Febrile neutropenia (FN) is among the leading causes of mortality and morbidity in hematology-oncologic patients undergoing intensive cytotoxic chemotherapy. It implies a large economic and social burden on the health system1,2 as it represents the most frequent complication in these patients.3 Infectious complications are the main cause of death not related to cancer progression.
The incidence of FN was reported in around 10–50% of patients with solid tumors and up to 80% of those with hematologic malignancies.4 In the pre-empiric antibiotics era, mortality due to infectious complications in patients receiving intensive chemotherapy was as high as 70%.5 Nowadays, this figure has dropped to between 1% and 18%,4,6,7 but still represents a serious problem that must be addressed actively using a multidisciplinary approach.
FN is a potentially life-threatening situation that requires prompt medical intervention. As neutropenic patients have an impaired inflammatory response, infection can occur with minimal signs and symptoms and progress rapidly, evolving with hypotension, renal failure, acidosis, or other life-threatening complications that lead to sepsis with multiorgan failure.2 As fever may constitute the isolated sign in these patients, it should be considered a real emergency. Early recognition of FN is critical to initiate broad-spectrum, empiric systemic antibacterial therapy promptly in order to avoid progression to sepsis and possible death.8
The prophylactic use of granulocyte colony-stimulating factor (G-CSF) to reduce the incidence of FN, as well as to enhance antibiotic therapy, has been widely studied in the last few years with conflicting results.9 In 2004 a Cochrane collaboration review concluded that the use of growth factors combined with antibiotic therapy in established FN caused by chemotherapy reduced the hospital stay and the duration of neutropenia, but the overall mortality was not influenced significantly.9 Furthermore, a meta-analysis in 2011 concluded that the use of G-CSF as primary prophylaxis reduced the incidence of FN in patients receiving chemotherapy for solid tumors and lymphoma.10
The management of FN is typically algorithm-driven. The effectiveness of the antibacterial protocol proposed by international guidelines to reduce FN-related mortality has already been reported.4,7 Thus, the aim of this study was to assess the impact of the implementation of international recommendations as the standardized protocol of local guidelines in 2011.11 One of the specific objectives of this study was to assess whether the protocol was correctly followed in each case.
MethodsThis is an analytic observational, retrospective, cohort study conducted from July 2011 to August 2012. The data were collected from the medical charts preserving the confidentiality of each patient.
PatientsThe inclusion criteria were patients older than 18 years, undergoing intensive chemotherapy in the Hematology–oncology Department of the Hospital de clínicas Dr. Manuel Quintela in Montevideo, Uruguay, for whom high-risk neutropenia was expected. Patients treated in this service that, because of their personal risk factors and comorbidities, suffered high-risk neutropenia but did not receive intensive chemotherapy were excluded.
DefinitionsIntensive chemotherapy was defined as chemotherapy regimens that cause high-risk neutropenia such as those used to treat acute myeloid leukemia, acute lymphoid leukemia, Burkitt lymphoma, and second lines for Hodgkin's and Non-Hodgkin's lymphoma. High-risk neutropenia was defined as one that is expected to last more than seven days.
Neutropenia was defined as a neutrophil count under 0.5×109/L or under 1.0×109/L when it was expected to reach under 0.5×109/L within the following 48h. Severe neutropenia was defined as a neutrophil count under 0.1×109/L. Patients diagnosed with acute leukemia were considered to have functional neutropenia even though they had neutrophil counts above 1.0×109/L. Fever was defined as an oral temperature above 38°C or a persistent temperature above 37.8°C.
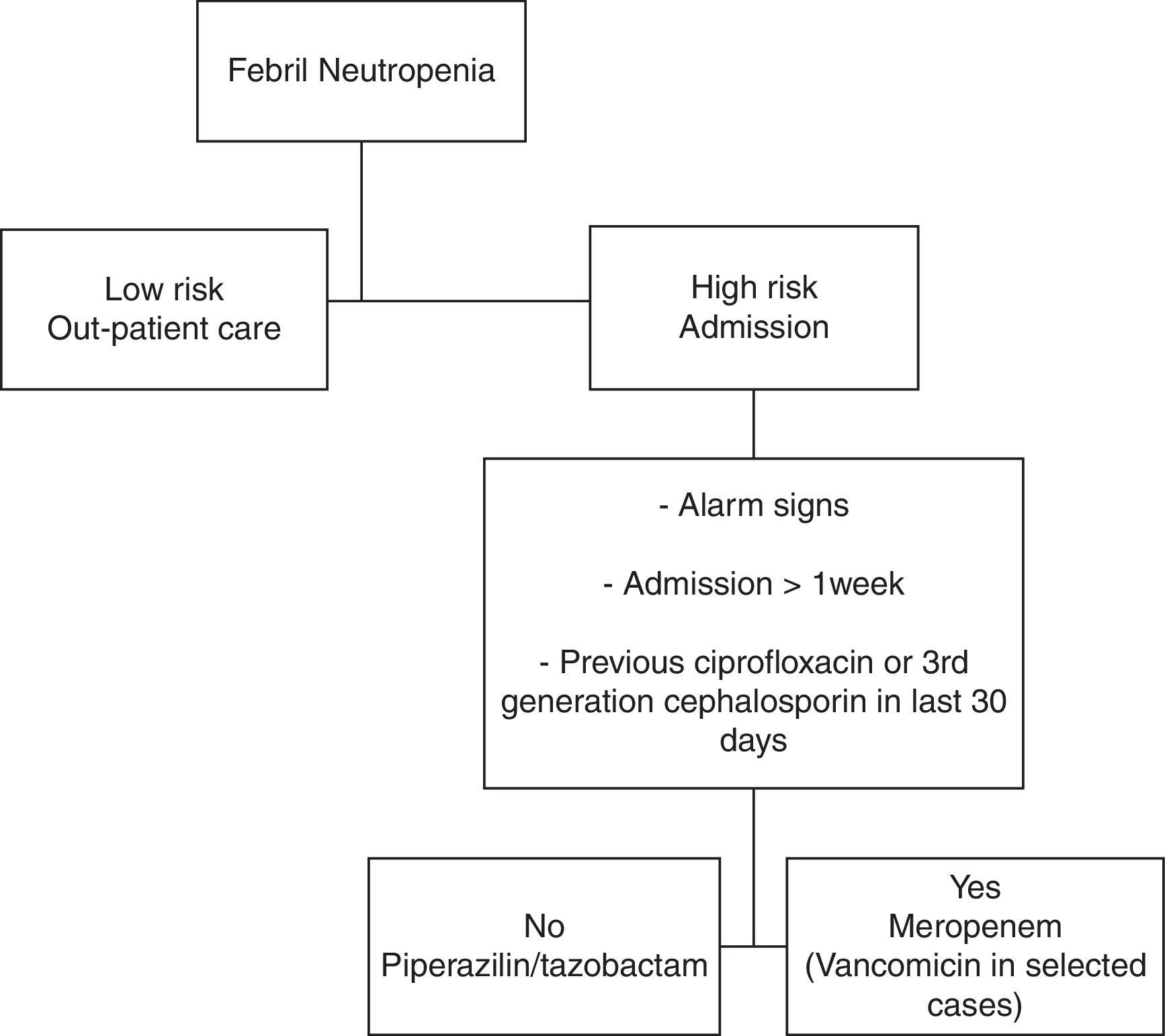
Alarm signs were defined in the protocol as the presence of at least one of the following: heart rate above 100 beats per minute, respiratory frequency above 20 breaths per minute, low carbon dioxide under 35mmHg, oxygen under 100mmHg or oxygen saturation under 93% while receiving supplementary oxygen, capillary refill longer than eight seconds, low pH, base excess under 5meq/L, serum lactate above 2mmol/L, systolic blood pressure under 90mmHg, confusion, or oliguria.
Clinical and laboratory studiesWhen a febrile episode was diagnosed, a detailed physical examination was made and repeated daily. Additionally, samples of blood, and urine and samples from other suspected infection sites were taken before the initiation of empirical antibiotic treatment. If the patient had a central venous catheter, at least one blood culture was prepared for each lumen of the catheter and one of a peripheral vein. A chest radiograph was obtained and urinalysis performed within the first 24h. Computed tomographies (CT) of the lung, head, sinuses, abdomen, and pelvis were performed as clinically indicated. Routine hematological investigations and biochemical analysis were carried out before treatment was started and every three days thereafter during the course of the therapy.
Additionally, C-reactive protein and procalcitonin levels were determined. A sinus and lung CT and serial galactomannan antigen test were performed prior to the initiation of antifungal therapy when a fungal infection was suspected in patients who remained febrile after 6–7 days of broad-spectrum antibiotic treatment.
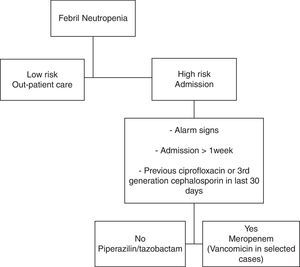
Antibiotic treatment protocolThe protocol consisted in the use of a broad-spectrum antimicrobial (Figure 1).11 Piperacillin–tazobactam therapy (4.5g every 6h) was started in patients without one of the following: alarm signs, more than one week of hospital stay, or having received ciprofloxacin or third-generation cephalosporin as prophylaxis within the previous 30 days. The other patients received meropenem (1g every 8h). Prophylactic antiviral (acyclovir) and antifungal (fluconazole) medications were given in all cases.
The initial empirical treatment was modified by changing the medication to meropenem, if there was: (a) deterioration in the clinical state after 24h, (b) alarm signs, hemodynamic instability or other organ dysfunction, (c) fever persistence after four days of treatment, (d) culture with antibiotic-resistant organism (particularly methicillin-resistant Staphylococcus aureus [MRSA] or extended-spectrum b-lactamase [ESBL]-producing gram-negative bacteria). In patients with hemodynamic instability, skin or soft tissue infection, suspected catheter-related infection or MRSA culture positive, vancomycin was added (1g every 12h). Vancomycin was stopped after two days if there was no evidence of gram-positive infection. Documented clinical and/or microbiological infections were treated with antibiotics appropriate for the site and susceptibility of each isolated organism. Empirical antifungal coverage was considered in patients who had persistent fever after 6–7 days of antibiotic treatment without identified fever source.
Statistical analysisStatistical analysis was made using the Statistics Program for Social Sciences software. The Chi-square test was used to compare categorical variables and the Mann–Whitney test for continuous variables. The Odds ratio was calculated. A p-value<0.05 was considered statistically significant.
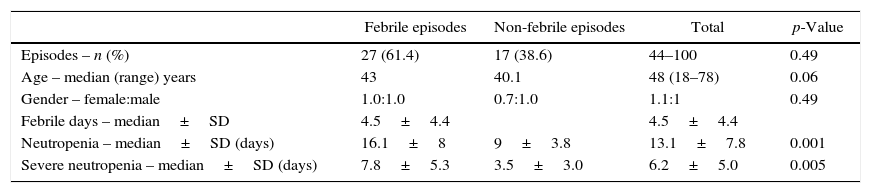
ResultsForty-four episodes of high-risk neutropenia (17 patients) with a median age of 48 years (range: 18–78 years) were included in this study (Table 1). There was a slight predominance of females (female:male ratio 1.1:1). The distribution of cancer types and chemotherapy treatment regimens are shown in Table 1. Acute myeloid leukemia (AML) and its treatment were the most common type of cancer and chemotherapy regimen. Non-Hodgkin's lymphomas comprising Burkitt's lymphomas (nine episodes registered in two patients), lymphoblastic lymphoma (one episode), entheropathy-associated T Lymphoma (one episode), and refractory or relapsed diffuse large cell lymphoma or T lymphomas (three episodes in two patients) were observed.
Patients’ characteristic.
| Febrile episodes | Non-febrile episodes | Total | p-Value | |
|---|---|---|---|---|
| Episodes – n (%) | 27 (61.4) | 17 (38.6) | 44–100 | 0.49 |
| Age – median (range) years | 43 | 40.1 | 48 (18–78) | 0.06 |
| Gender – female:male | 1.0:1.0 | 0.7:1.0 | 1.1:1 | 0.49 |
| Febrile days – median±SD | 4.5±4.4 | 4.5±4.4 | ||
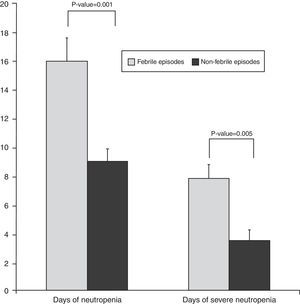
| Neutropenia – median±SD (days) | 16.1±8 | 9±3.8 | 13.1±7.8 | 0.001 |
| Severe neutropenia – median±SD (days) | 7.8±5.3 | 3.5±3.0 | 6.2±5.0 | 0.005 |
| Hematology–oncology diagnosis | n | (%) | n | (%) | n | (%) |
|---|---|---|---|---|---|---|
| AML/myelodysplastic syndromes | 18 | 66.6 | 7 | 41.2 | 25 | 56.8 |
| Acute lymphoid leukemia | 2 | 7.5 | 1 | 5.9 | 3 | 6.8 |
| Non-Hodgkin's lymphoma | 7 | 25.9 | 7 | 41.2 | 14 | 31.9 |
| Hodgkin's lymphoma | 0 | 0 | 2 | 11.7 | 2 | 4.5 |
| Chemotherapy regimen | n | (%) | n | (%) | n | (%) |
|---|---|---|---|---|---|---|
| High-dose cytarabine (HIDAC) | 6 | 24.0 | 7 | 41.2 | 13 | 29.5 |
| Cytarabine–Daunorubicin (7+3) | 8 | 32.0 | 0 | 0 | 8 | 18.2 |
| CODOX-M | 4 | 16.0 | 3 | 17.6 | 7 | 15.9 |
| IVAC | 1 | 4.0 | 2 | 11.8 | 3 | 6.8 |
| FLAG | 2 | 8.0 | 0 | 0 | 2 | 4.5 |
| ESHAP | 0 | 0.0 | 2 | 11.8 | 2 | 4.5 |
| MINI BEAM | 0 | 0.0 | 2 | 11.8 | 2 | 4.5 |
| Berlin–Frankfurt–Munich (BFM) 2008 protocol | 1 | 4.0 | 1 | 5.8 | 2 | 4.5 |
| Hyper-CVAD | 1 | 4.0 | 0 | 0 | 1 | 2.3 |
| IVE | 1 | 4.0 | 0 | 0 | 1 | 2.3 |
| HIDAC+Daunorubicin | 1 | 4.0 | 0 | 0 | 1 | 2.3 |
AML: Acute myeloid leukemia; CODOX-M: cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate; IVAC: ifosfamide, etoposide and high-dose cytarabine; FLAG: fludarabine, cytarabine, and filgrastim; ESHAP: etoposide, methylprednisolone, cytarabine, and cisplatin; MINI BEAM: carmustine, etoposide, cytarabine, and melphalan; Hyper-CVAD: cyclophosphamide, vincristine, doxorubicin, and dexamethasone; IVE: ifosfamide, vincristine, and etoposide
Of the 44 episodes of high-risk neutropenia, 27 (61.4%) experienced fever during neutropenia. Every patient included in this study experienced FN in at least one of the episodes.
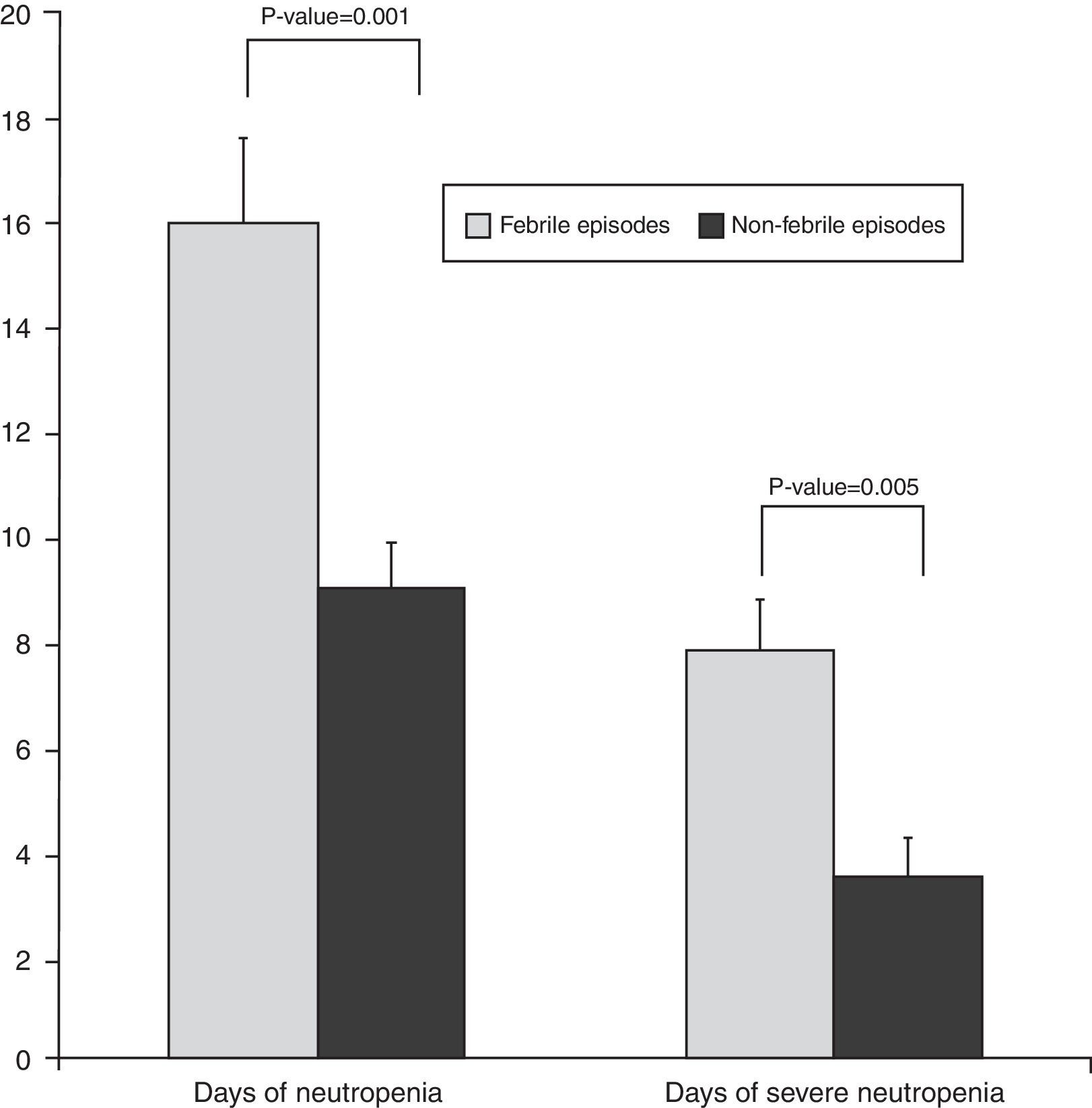
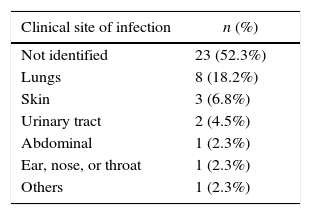
According to the results, FN was significantly associated with both the duration of neutropenia (p-value=0.001) with a median of nine days for afebrile vs. 16 days for febrile episodes, and the duration of severe neutropenia (p-value=0.005) with a median of 3.5 days for afebrile compared with 7.8 days for febrile episodes (Figure 2). The prophylactic use of filgrastim was not standardized in our service and depended on the choice of each physician. The use of filgrastim was associated with neither the duration nor the incidence of FN. In 62% of the episodes, the site of infection was identified, either clinically, by imaging techniques, or by microbiological cultures. As shown in Table 2, the respiratory tract was the most common site of infection (41%), followed by the skin and others.
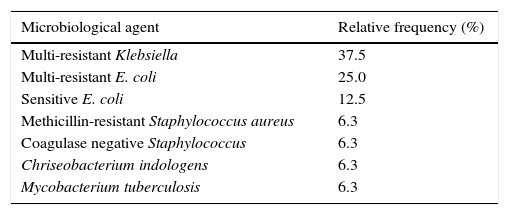
Microbiological cultures were achieved in 59.3% (n=16) of the febrile episodes (Table 2). A total of 75% of all isolates were gram-negative bacilli (GNB). Of the multiple-antibiotic-resistant (MR) GNB (62.5%), defined as resistance to at least three different antibiotic groups, six isolates were MR Klebsiella pneumoniae (specifically ESBL K. pneumonia) and four isolates were MR Escherichia coli (ESBL). Regarding the source of the isolate (Table 3), 13 were from blood cultures, representing 48% of bacteremia in all FN episodes and 81.3% of all microbiological documented infections. All of the MR-GNB were susceptible to imipenem, meropenem and amikacin and resistant to all other antibiotic classes, including piperacillin and tazobactam.
Microbiological isolation.
| Microbiological agent | Relative frequency (%) |
|---|---|
| Multi-resistant Klebsiella | 37.5 |
| Multi-resistant E. coli | 25.0 |
| Sensitive E. coli | 12.5 |
| Methicillin-resistant Staphylococcus aureus | 6.3 |
| Coagulase negative Staphylococcus | 6.3 |
| Chriseobacterium indologens | 6.3 |
| Mycobacterium tuberculosis | 6.3 |
When considering only the first FN episode of each patient, microbiological isolates were identified in 53% of episodes with 77.7% of these being MR-GNB. For 14 (63%) of the episodes in which the initial treatment was piperacillin/tazobactam, therapy needed to be escalated to meropenem in a mean time of 4.5±2.5 days of treatment. The mortality rate of these episodes was 28%; 47% had a MR-GNB and 35% had negative cultures. One patient was diagnosed with pulmonary tuberculosis.
The overall mortality rate in all neutropenic episodes was 13.6% (n=6) and the mortality rate in febrile neutropenic episodes alone was 18.5%. Three of these patients died due to sepsis (11.1% of mortality due to sepsis in FN episodes) and the others due to disease progression. Every death-related infection was reported in the first FN episode. Death occurred in 35% of the episodes in which the microbiological agent was isolated and there were no deaths in episodes with negative cultures (p-value=0.027).
Nine (33%) of the FN episodes presented at least one of the alarm signs defined in the protocol of this study. The mortality rate of these was 44% vs. 6% in episodes without alarm signs (p-value=0.018). This determines an OR of 13.0 for the presence of alarm signs.
There were 15 (34.1%) episodes that were treated with piperacillin–tazobactam as front-line therapy even though these patients had received prophylaxis with ciprofloxacin.
DiscussionInfectious diseases are an important complication in hematology-oncologic patients resulting in longer hospital stays, and increased morbidity and mortality. Neutropenia has been recognized for many decades as a major risk factor for the development of infections in hematology-oncologic patients undergoing chemotherapy.12
Here, we present the first-year results and features of infectious diseases since the implementation of a new protocol in the management of FN in high-risk hematology-oncologic patients at a University Hospital in Uruguay. Before the implementation of this protocol the management of FN was not standardized in this service and physicians decided based on the available evidence, the international guidelines, and their own personal experience.
The main findings of this work were the high rate of microbiological agents isolated in FN episodes, and the elevated prevalence of MR-GNB.
Microbiological documented infections were statistically associated with higher mortality. This finding can be explained by the fact that bacteremia was the most frequent documented infection (81.3%). Teixeira et al. recently reported that in hematopoietic stem cell transplantation patients, microbiologically documented infections represented a death risk factor and that bacteremia was the most commonly documented infection (46.3%).13 Furthermore, it is known that in other infectious diseases, such as community-acquired pneumococcal pneumonia, bacteremia is associated with increased severity and mortality.14
The etiology of infections in FN has varied in the last fifty years. In the 1970s and early 1980s there was a predominance of gram-negative microorganisms but in the late 1980s and in the 1990s there was a dramatic increase in gram-positive bacteria, with these becoming the most common infecting organisms.15,16 This led to changes in antibiotic treatments, focusing on resistant gram-positive strains.17 However, in the last few years, an increase in GNB has been reported worldwide.16 This work identified an important predominance of GNB concordant with other recent regional reports.14,15
However, there were more resistant GNB, particularly ESBL, than reported in most series.13,15,16
There was an early need to escalate to meropenem in a high number of episodes, and in many of them MR-GNB were isolated.
The mortality rate observed due to sepsis was 23.5% and this was statistically associated with the isolation of microbiological agents and the presence of at least one alarm sign.
The incidence of FN in our service was similar to others reported in the literature.4,6,7 However, the mortality rates were slightly higher than reported by referral services but similar to those reported in other Latin American countries.18,19
The presence of fever was associated with duration and severity of neutropenia. Historical studies show that as the neutrophil count drops below 0.5×109/L, the susceptibility to infection increases.20 Moreover, it has been reported that the frequency and severity of infection are inversely proportional to the neutrophil count. The risk of severe infection and bacteremia are high when the neutrophil count is less than 1.0×109/L. The rate of decline of the neutrophil count and the duration of neutropenia are also important factors to consider.21–23 Additionally, it has been reported that an increase in the neutrophil count during treatment improves outcomes. Bodey et al. informed that the mortality rate was higher (80%) among patients who initially started with neutrophil counts below 1.0×109/L that did not rise during the first week of infection compared to the mortality rates (27%) seen in patients whose neutrophil counts rose above 10.0×109/L.21
Hematopoietic growth-stimulating factors are a class of cytokines that regulate proliferation, differentiation, and functions of hematopoietic cells. G-CSF regulates neutrophil production.24 The administration of G-CSF to humans results in a dose-dependent increase in circulating neutrophils.24 In this study, a significant reduction in the incidence of FN using filgrastim was not found, contrary to what was expected according to the literature.9,10 This result may be due to the small sample size. Most of the episodes (77%) were treated using filgrastim. This result should be re-analyzed with more episodes.
The protocol was not adequately followed in every case. Although this may represent a limitation when interpreting the findings of this study, detecting these kinds of failures is important in order for them to be corrected.
This study emphasizes the high isolation rates of microbiological agents, especially GNB resistant to piperacillin/tazobactam, which constitute the front-line antibiotics in our protocol. The early need of escalation to carbapenems raises the question as to whether these should be the front-line treatment for high-risk neutropenia patients in our service. Moreover, this is a big step as carbapenems are at the top of the antibiotic option list and when used as first-line treatment, many problems with resistant strains would probably arise. As alternatives to carbapenem, a combination of piperacillin–tazobactam and amikacin may be an effective empirical therapeutic option for patients with neutropenic fever who are at high risk of developing bacteremia with ESBL-producing pathogens.
We believe that a larger study should be conducted before making a final decision because, although the present work represents one year's experience of the evolution of high-risk neutropenia at a university hospital, the number of the analyzed episodes is too small to conclude that our front-line antibiotic option is not suitable.
ConclusionsIn this work we observed a high isolation rate of microbiological agents in FN episodes; this was statistically associated with higher mortality. Bacteremia was the most common microbiological isolate identified with a predominance of GNB, particularly MR. Risk factors for FN were duration and severity of neutropenia and the isolation of a microbiological agent, and the presence of alarm signs was associated with poor outcomes. The high rate of GNB resistant to piperacillin/tazobactam, the front-line antibiotics in our protocol, and the early need to escalate to carbapenems raises the question as to whether it is necessary to change our antibiotic treatment protocol for high-risk neutropenia. Further prospective studies with a larger number of patients and episodes of FN should be conducted to confirm these results.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank all those who participated in the development and implementation of the febrile neutropenia therapeutic protocol: Infectious Diseases Department, Bacteriology (Clinical Laboratory Department), Pharmacology Department and Intensive Care Medicine Department.