Renal infarction is an infrequent condition resulting from obstruction of a renal artery. The symptoms it causes are non-specific and as a result the diagnosis may be missed, resulting in loss of renal parenchyma, or the risk of other thrombotic phenomenon. The estimated incidence of renal infarction, based on emergency service admissions, is between 0.004 and 0.007%.1 Four risk groups for renal infarction have been suggested: renal infarction of cardiac origin, the most common of which is atrial fibrillation; renal infarction associated with renal artery injury; renal infarction associated with thrombophilia, and; finally, idiopathic. The SARS-CoV-2 infection (Covid-19) has been associated with an increased risk of both arterial and venous thrombosis. A meta-analysis reported an incidence of venous thrombosis in 22.7% of Intensive care unit (ICU) patients and 7.9% in non-ICU patients2 and reports of an increased risk of arterial thrombosis in severely ill patients, even in those patients receiving antithrombotic prophylaxis with low molecular weight heparin.3,4 Arterial thrombosis has involved the cerebral, coronary, the aorta and peripheral arteries.3,4 In a report on 241 patients with severe COVID-19, 5.7% suffered from acute ischaemic cerebrovascular disease.3 However, there is limited evidence of the risk of both arterial and venous thrombosis in patients with asymptomatic COVID-19.
In this context of asymptomatic patients, we present the case of a previously healthy 25-year-old man with no known risk factors for thrombosis who presented to the emergency services with severe loin pain and had a positive polymerase chain reaction (PCR) test for SARS-CoV-2 infection. The angio-computed tomography (CT) of the abdomen and pelvis showed images consistent with thrombosis in one of the two left renal arteries, associated with renal infarction. We present the clinical and laboratory findings, along with the patient's management.
Clinical caseA 25-year-old man presented with a two-day history of sudden onset, increasing left loin pain, associated with fever. He had no other symptoms, no previous medical history of thrombotic risk factors, was a non-smoker, was taking no regular medication and had taken ibuprofen for the pain. There was no family history of thrombotic disorders. Clinical examination showed normal vital signs, normotensive, with a low-grade fever of 37.7°C. Examination showed tenderness in the left loin region but no other findings. Blood tests revealed a leucocytosis of 15,900 leukocytes/µl, with a neutrophilia of 11,500 neutrophils/µl, an elevated erythrocyte sedimentation rate of 50mm/hour (normal < 20mm/hour), an elevated C-reactive protein of 91.45 (normal range < 3.0mg/l), a normal creatinine of 0.78mg/dl (normal range 0.7 - 1.2mg/dl) and a calculated glomerular filtration rate of 129mls/min/m2, an increased LDH of 725U/l (normal range < 250U/l), the prothrombin and activated partial thromboplastin times (APTTs) were normal, as was the platelet count. A complete urine test showed no evidence of microscopic or macroscopic hematuria and urine culture was negative for bacterial growth. Both the D-dimer and fibrinogen levels were slightly elevated on admission, D- dimer 502ng/ml (day 1) increasing to a maximum of 585ng/ml (day 3) (normal < 500ng/ml) and fibrinogen 495mg/dl (day 1) rising to a maximum of 563mg/dl (day 4) (normal range 200 - 400mg/dl). The ferritin level was normal throughout the hospital stay.
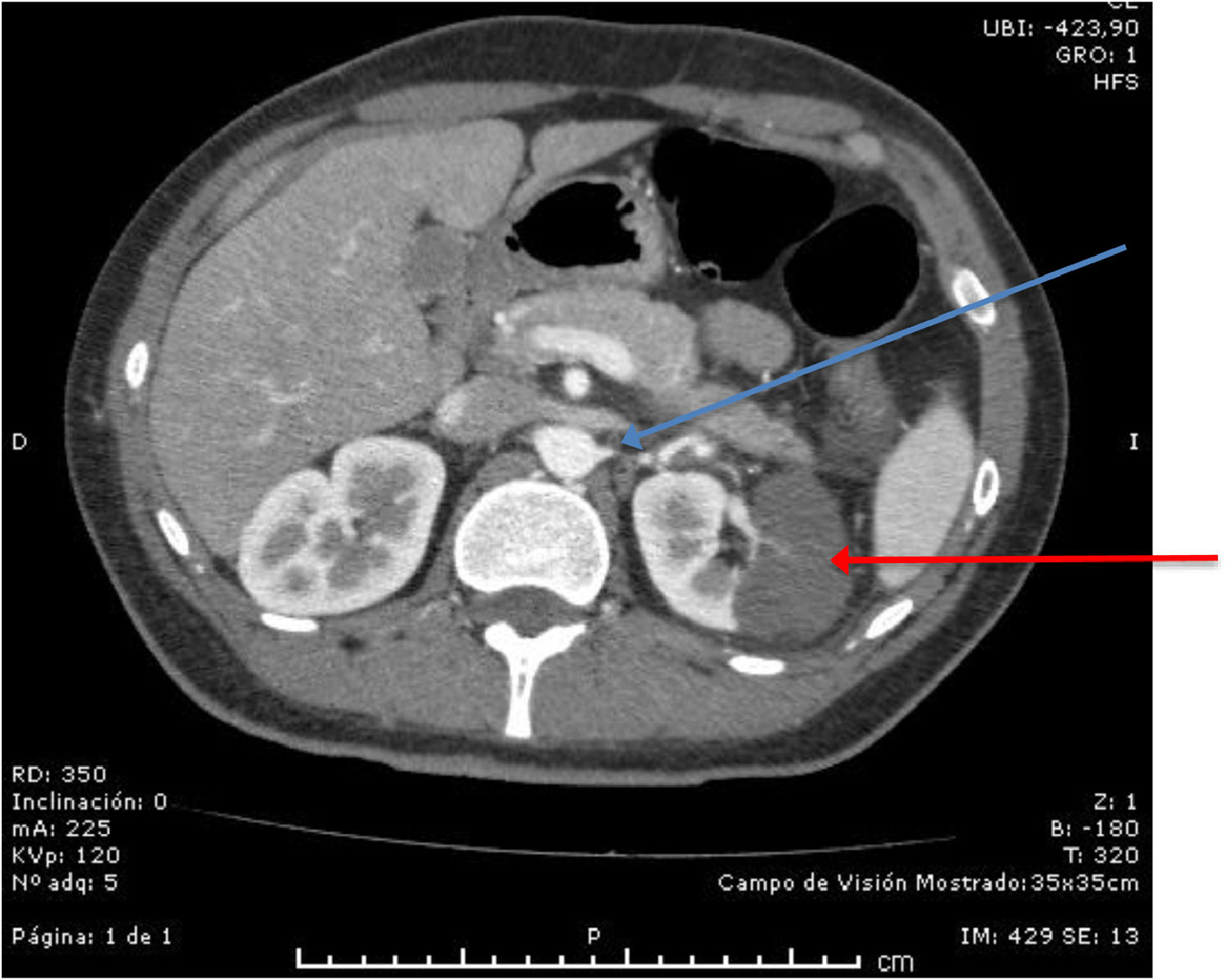
The angio-computed tomography (CT) of the thorax, abdomen and pelvis showed images consistent with a wedge-shaped infarct of the lateral cortex of the left kidney associated with a thrombosis in one of two left renal arteries associated with the area of the renal infarct (Figure 1). The rest of the angio-CT was normal, except for sub-segmental atelectasis in the base of the left lung. The electrocardiogram did not show atrial fibrillation and an echocardiogram showed no cardiac source of an embolus.
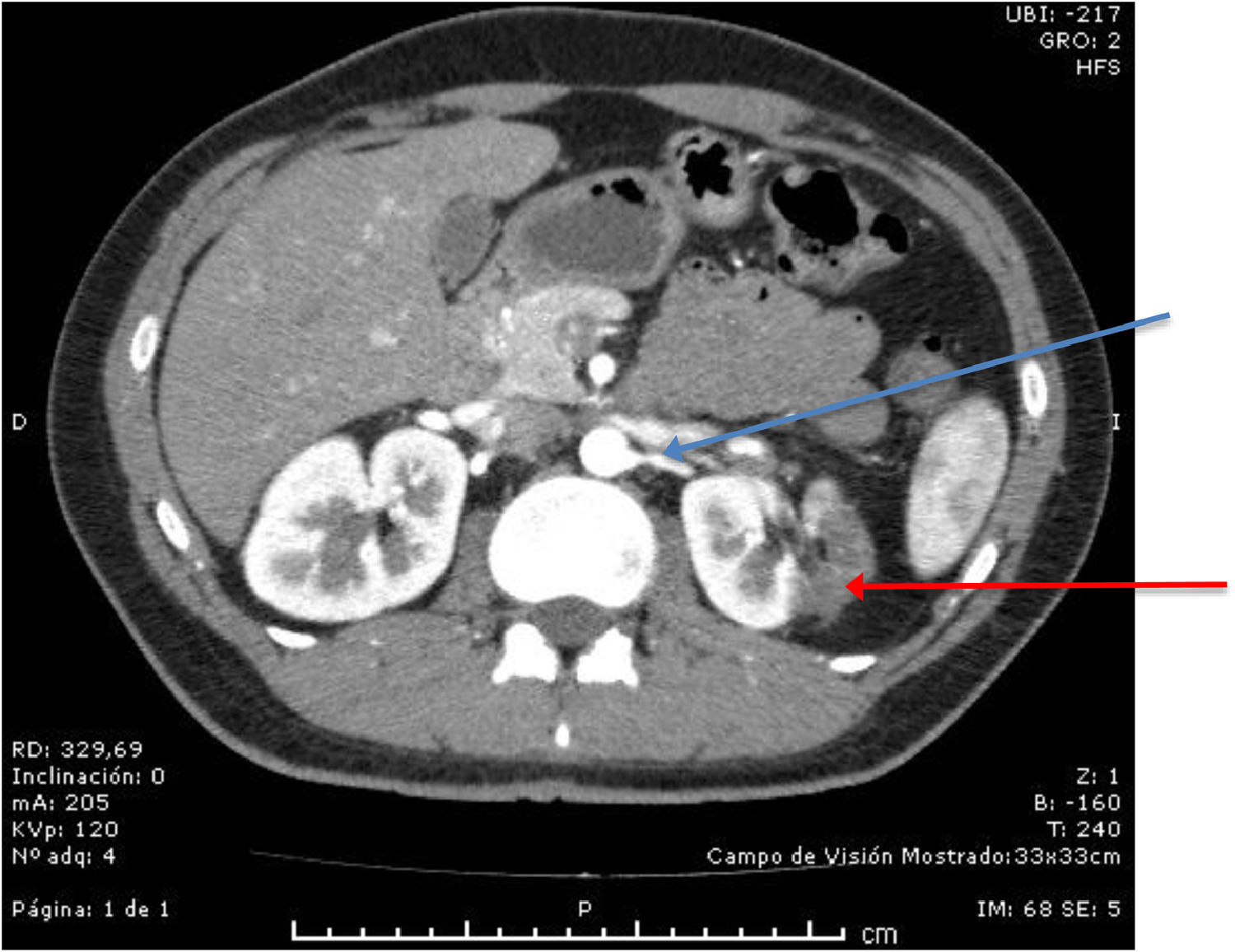
All patients needing hospitalization are tested for SARS-CoV-2, using the PCR, which was positive for this patient. In the Emergency Department, blood samples were taken to exclude a thrombophilia. The lupus anticoagulant, anticardiolipin antibodies and anti-beta-2-glicoprotein antibodies were all negative, excluding the anti-phospholipid syndrome. The levels of protein C, S and anti-thrombin III were normal and the tests for Factor V Leiden and the gene for prothrombin mutation were negative. The patient was started on 12-hourly s/c dalteparin at 7,500 IU, analgesia and referred to the Hematology Department. The patient was evaluated by the Hematology and Urology teams and the anticoagulant was changed to 12-hourly oral rivoxaban at 15mg for 21 days, followed by 20mg daily, until completing six months of therapy. The patient remained normotensive during the hospitalization and the creatinine remained in the normal range. A follow-up angio-CT scan after three months of anticoagulation showed re-canalization of the renal artery (Figure 2).
With respect to the COVID-19 infection, the patient remained asymptomatic, did not require oxygen support, intensive care unit management or mechanical ventilation. He was discharged after 8 days.
DiscussionReports of arterial thrombosis in patients with COVID-19 have varied; a retrospective observational study of 1,419 patients reported 1% of patients with arterial thrombosis,5 while more recently, 30% of the patients with severe COVID-19 had major adverse cardiovascular events and 23% of these had an early failure of percutaneous coronary interventions or lower limb surgical revascularization.6
The underlying mechanisms of COVID-19-induced thrombosis are largely unknown and several mechanisms have been suggested, including a direct cytotoxic effect of SARs-COV-2 on vacular endothelial cells. The virus invades endothelial cells via the angiotension-converting enzyme 2 receptor, which is found on endothelial cells.7 Viral replication causes an inflammatory cell infiltrate, apoptosis of the endothelial cells and a microvascular prothrombotic state with micro-circulatory dysfunction and thrombosis, which leads to vasoconstriction and end-organ ischemia. Secondly, in patients with severe COVID-19 there is an increased production of inflammatory cytokines, such as Interleukin-1, 2 and 6, along with tumor necrosis factor alpha. This results in platelet and neutrophil activation, endothelial damage and thrombosis7 Thirdly, the activation of monocytes in severe COVID-19 upregulates the tissue factor, leading to the activation of the coagulation cascade and thrombosis, while that of neutophils leads to extracellular trap generation, activating the cytokine storm and thrombosis. As a result, in patients with severe COVID-19, this inflammatory response is reflected in the significant increases in the eythrocyte sedimentation rate (ESR), C-reactive protein, ferritin and D-Dimer levels.7 Contrary to the literature, in this clinical case, the patient was asymptomatic, with only slightly elevated levels of D-Dimer and fibrinogen. In the absence of risk factors for arterial thrombosis or emboli, thrombosis caused by COVID-19 appears to be the most probable explication in this case.
Differing from severely ill COVID-19 patients, in mild-to-moderately ill patients with arterial thrombosis, the majority of the patients have been reported to have a normal D-dimer and prothrombin times, but an elevated C-reactive protein, as was seen in this reported case.
With regards to thrombophilia screening in young patients with arterial thrombosis, inherited thrombophilias are associated with an increased risk of venous thrombosis, but their association with an increased risk of arterial thrombosis is less well established. The Anticoagulation Forum recommended that screening for thrombophilia should not be carried out in the setting of acute thrombosis or if the patient is taking anticoagulants, except for genotype-based tests (Factor V Leiden and prothrombin gene mutation) and antibody titres (cardiolipin and beta-2-glycoprotein).8 In terms of arterial thrombosis, the presence of anti-phospholipid antibodies is a known risk factor and their presence has been reported in severely ill patients with COVID-19, anti-beta-2-glycoprotein in up to 15% of the cases and anti-cardiolipins in up to 7%.9 However, functional assays for anti-phospholipid antibodies are affected by the presence of heparin, direct inhibitors of Factor Xa and severe inflammation characterized by high levels of C-reactive protein.9 Furthermore, the association with thrombosis, both arterial and venous, in patients with COVID-19 has not been confirmed9
The treatment for renal artery thrombosis consists of anticoagulation and unfractionated heparin Is one alternative, but in patients with severe COVID-19 there may be a pseudo-heparin resistance. Monitoring with the APTT suggests resistance to heparin, with increased doses being needed to achieve therapuetic levels. This is due to elevated Factor VIII levels caused by systemic inflammation and, as such, activated FX levels should be used to monitor heparin dosage. The use of low molecular heparins, such as enoxaparin or dalteparin, can be used as alternates, with a once- or twice-daily subcutaenous doage and do not require monitoring. The use of vitamin K antagonists, the direct thrombin inhibitor dabigatrin and the factor Xa inhibitors apixaban and rivaroxaban should be considered with caution. Patients with severe COVID-19 are frequently treated with multiple drugs, including anti-virals, azitromycin and anti-interleukin-6, which all may increase direct anticoagulant levels in the plasma. It has been suggested that in clinically stable patients, such as in this case, vitamin K antagonists and direct oral anticoagulants may be considered or continued . However, in patients who need to be admitted to the ICU or at risk for a significant drug-drug interaction, these anticoagulants should be changed to low molecular weight heparin.10 We used rivaroxaban in this case, as ICU care was not necessary, there was no risk of drug interactions and this particular drug does not require monitoring during the six months of treatment. In times of the SARs-Cov-2 pandemic, this is an important consideration to reduce the risk of infection of health professionals and other patients attending the anticoagulant outpatient department.
ConclusionsPatients with mild forms or asymptomatic COVID-19 may be at risk for arterial thrombosis. This may be so, even in those patients without predisposing factors for arterial thrombosis.
Parameters that are frequently elevated in severe COVID-19 may be in the normal range in patients with mild or asymptomatic COVID-19, but this does not exclude the risk of thrombosis.
Screening for thrombophilia should be according to guidelines and not in the acute phase of thrombosis.
The use of direct oral anticoagulants in non-ICU patients with thrombosis should be considered, taking into consideration possible drug interactions.
This report highlights that SARs-COV-2 infection should be considered in young patients presenting with unusual arterial thrombosis.
Ethical considerationsThe patient provided written informed consent for the case report and this report was in complete accordance with the Declaration of Helsinki and Chilean Law on patient rights.
The authors wish to thank Mrs. Ana Maria Palazuelos for her help in the writing of this manuscript.