Mycosis Fungoides (MF) is the most common form of cutaneous T cell lymphomas, it accounts for almost 50% of all primary cutaneous lymphomas and Sezary syndrome (SS) is a leukemic variant associated with erythroderma.1 Advanced stages of MF/SS are associated with median survivals from approximately 1 to 5 years.2 For these patients, allogeneic hematopoietic cell transplant (allo-HCT) represents a potentially curative treatment option.3–5 A haploidentical donor is a valuable alternative for patients without HLA-matched donors and The European Society for Blood and Marrow Transplantation has evidenced comparable outcomes between haploidentical HCT (haplo-HCT) and HLA-identical-HCT recipients with non-Hodgkin lymphoma6; nevertheless, reports on patients with MF/SS are sparse.7,8 Moreover, available experience with reduced-intensity conditioning (RIC) haplo-HCT in the outpatient setting is scarce; yet, this deserves further investigation since availability of an HLA-matched donor can be a barrier to transplant and most importantly, given the radical changes adopted by worldwide health professionals in the way their care is delivered to lessen the COVID-19 pandemic burden. In the context of significant lifestyle adjustments of millions of hematology patients intended to reduce the risk of the virus’ spread and the fear of contact with healthcare facilities, we report a case of advanced stage, refractory MF/SS successfully treated with RIC haplo-HCT with post-transplant cyclophosphamide (PTCy) on a completely outpatient basis.
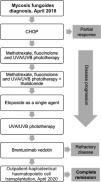
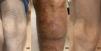
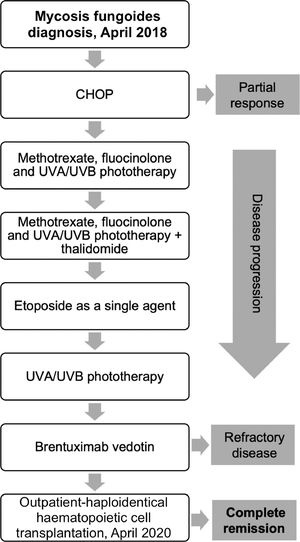
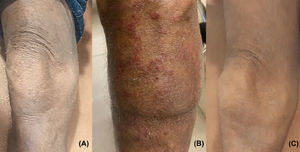
Case reportThe patient is a 69-year-old male with a 20-month history of pruritic patches and plaques on his trunk, both hands and feet. He was diagnosed in April 2018 with biopsy-confirmed stage IIIA T4N1 MF, documenting enlarged epidermotropic lymphocytes with irregular nuclei; immunohistochemistry examinations showed CD5+, CD4+, CD3+, CD2+ and CD30–. An initial computed tomography scan (CT) revealed bilateral involvement of axillary and inguinal nodes. A summary of the treatments received by the patient is presented in Figure 1, he began treatment with CHOP, however only a partial response was achieved; subsequently he received weekly methotrexate, fluocinolone and UVA/UVB phototherapy with minimal response; thalidomide was added with no response, the patient later received weekly single-agent etoposide with no improvement. Over the following months, he progressed with diffuse erythroderma and scaly-pruritic multifocal lesions affecting more than 50% of the body surface area (BSA) which were treated with UVA/UVB phototherapy three times a week; he obtained pruritus relief and stable disease. On January 2020, the patient initiated brentuximab therapy (1.8 mg/kg, four cycles); however, he developed grade 2 peripheral neuropathy after the first cycle with no improvement after using pregabalin 75 mg orally twice daily and consequently the dose of the fourth cycle had to be decreased. Up to this point the patient persisted with refractory disease involving peripheral blood and >40% of the BSA with fissuring and cracking of the skin (Figure 2a). A peripheral blood flow cytometry showed an atypical CD3++ CD4+ CD5+ CD30– and partially expressed CD7 T-cell population. On April 2020, he was evaluated by our transplant team as candidate for allo-HCT, the benefits and potential risks of a HCT were explained to the patient given the conditions imposed by the COVID-19 outbreak; he agreed to receive the procedure on a fully outpatient basis, including placement of central venous access, fluid infusion, conditioning, graft infusion and the aplastic phase.9 He did not have a matched donor thus his son was selected as a haploidentical donor match. The patient began his RIC conditioning regimen which consisted of intravenously (i.v.) fludarabine 25 mg/m2/day on days –3, –2 and –1; i.v. cyclophosphamide 350 mg/m2/day on days –2 and –1; oral melphalan 100 mg/m2/day on day –1 and 200 cGy of total body irradiation on day –1. Graft‐versus‐host disease (GvHD) prophylaxis consisted of PTCy 50 mg/kg, oral cyclosporine A and mycophenolate mofetil at standard doses. Time from diagnosis to transplantation was 24 months. Infusion of 9.5 × 106 CD34+/kg from peripheral blood occurred on day 0 without any adverse event, graft infusion was performed over 15 min and after a one-hour observation period the patient was sent home where he remained under the supervision of his caregiver who was a family member previously instructed by the treating physician; signs of events requiring immediate attention were explained by the treating physician, including 24-h contact phone numbers for receiving urgent attention at any time and day. During the first month post-transplantation, staying at home following stringent hygiene measures was required during the first four post-transplant weeks; after this period the patient was allowed to freely continue with his daily activities; he had daily follow-up visits to the hematology day clinic from day +1 until hematological recovery was observed, subsequently every 48 h until day +30 and then weekly until day +90 and once a month thereafter; our complete method has been previously described.9 Grade I mucositis was documented on day +5 which progressed to grade II mucositis on day +11 and later resolved. He received one packed red blood cells unit on day +7 and one plateletpheresis on day +11. Platelet and neutrophil engraftment occurred on day +13. The patient was CMV positive at baseline and reactivation occurred which was successfully treated with valganciclovir. At day +100 donor chimerism was 100% and a complete response was achieved as demonstrated by bone marrow and peripheral blood testing; his CT scan showed remission of the lymphadenopathy previously identified and the skin exam evidenced achievement of soft skin with no erythroderma, cracks or fissures. On day +27 the patient developed pruritic cutaneous lesions on his lower extremities (Figure 2b) and a skin biopsy confirmed GvHD which later progressed to steroid-refractory grade II GvHD, hence, he initiated rituximab therapy on day +153 and concluded on day +187, a total of 4 doses of 100 mg were administered i.v. and the disease remitted at the end of this treatment (Figure 2c). Despite these post-HCT complications, the patient tolerated transplant well, he did not present neutropenic fever or infection and completed transplantation without hospitalization. Following haplo-HCT the patient achieved complete remission, demonstrated full chimerism and he is disease-free 36 months from diagnosis and 11 months after transplant with excellent quality-of-life.
Summary of treatments received by a 69-year old male for mycosis fungoides/Sezary syndrome. Front-line chemotherapy consisted of CHOP, achieving partial response. Fluocinolone, UVA/UVB phototherapy, methotrexate, thalidomide and etoposide were administered, without response. Complete remission was achieved after haploidentical hematopoietic cell transplantation.
After receiving brentuximab, the patient persisted with refractory mycosis fungoides/ Sezary syndrome involving the skin, with mild cracking and fissuring (a). He underwent outpatient-haploidentical hematopoietic cell transplantation. On day +27 the patient developed erythematous pruritic cutaneous lesions on his lower extremities and a skin biopsy confirmed acute graft‐versus‐host disease (GvHD) which later progressed to steroid-refractory GvHD (b). The patient received rituximab successfully from day +153 to +187; he completed the transplant entirely as an outpatient, remained in complete remission and showed no further evidence of GvHD at eleven months after haploidentical cell transplantation (c).
Although the likelihood of relapse, GvHD, infection or death can discourage allo-HCT, this malignancy's changing therapeutic panorama leads us to consider its role in offering a potential cure; additional research on the optimal timing, donor type, conditioning regimen and GvHD prophylaxis is essential.
To our knowledge, we report the first case of a fully ambulatory RIC haploidentical hematopoietic cell transplantation for mycosis fungoides/Sezary syndrome carried out during the COVID-19 pandemic. Based on our patient's outcome we believe outpatient RIC haplo-HCT for advanced MF/SS provides an important graft-versus-lymphoma effect and should be considered a viable option for eligible patients without an HLA-matched donor. Our institution's outpatient-based transplant program has allowed us to continue performing hematopoietic cell transplants during the current pandemic, contrary to halting of the procedure in other programs.10,11 Thus, healthcare systems and transplant centers must be strengthened to meet the needs of patients in whom haplo-HCT represents the only plausible curative strategy.
Our case adds evidence that the management of hematology patients should not have been suspended or postponed in the COVID-19 era, in which a rebound effect overstressing transplant programs was anticipated and occurred12; thus offering an outpatient haploidentical transplant for patients with hematological malignancies is feasible, safe, cost-effective and potentially life-saving.
We thank Karla Paz-Guizar, MD., for providing clinical images and Sergio Lozano-Rodriguez, M.D., for his help in editing the manuscript.