The emergence of resistance has been demonstrated in cancer treatment centers where prophylaxis with fluoroquinolone is used.
ObjectiveConsidering the importance of epidemiological monitoring as a strategy in choosing protocols involving antibiotics, this study aimed to evaluate the emergence of quinolone resistance and changes in the local epidemiology in a hematopoietic stem cell transplant service.
MethodsFor this study, 60 positive cultures before the prophylactic use of levofloxacin (period A: 2007-2008) and 118 cultures after starting the use of prophylactic levofloxacin (period B: 2010-2011) were evaluated.
ResultsResistance increased for all the different types of bacteria isolated (from 46.0% to 76.5%; p-value=0.0002). Among Gram-negative bacteria, resistance increased from 21.4% to 60.7% (p-value=0.0163) and among Gram-positive bacteria, it increased from 55.6% to 82.9% (p-value=0.0025). The use of levofloxacin increased from 19.44 defined daily doses per 1,000 patient-days in period A to 166.64 in period B. The use of broad spectrum antibiotics remained unchanged. Considering bacteria associated with infection, 72 and 76 were isolated in periods A and B, respectively. There was a reduction in the rate of Gram-negative bacteria in cultures associated with infection (3.81 vs. 2.00 cultures/1,000 patient-days; p-value=0.008).
ConclusionThe study of prophylaxis with levofloxacin demonstrated that there was a decrease in infections by Gram-negative bacteria; however, bacterial resistance increased, even though the use of broad-spectrum antibiotics remained unchanged. Constant monitoring of local epidemiology combined with research on clinical outcomes is needed to evaluate the effectiveness of prophylaxis.
© 2014 Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. All rights reserved.
Patients undergoing hematopoietic stem cell transplantation (HSCT) during the neutropenic period have a high risk of fever, with a reported occurrence greater than 80%.1
In an attempt to decrease the risk of associated complications, it has been routine for several decades to use antimicrobials as clinical prophylaxis during the neutropenic phase of HSCT patients. Fluoroquinolones were introduced in the 1980s as a prophylactic agent, and since then, this has been the most commonly used antibiotic class prescribed in this setting.2
Some aspects of the benefits brought by prophylactic fluoroquinolone use are still questioned. Reductions in the time of febrile neutropenia and infections in neutropenic patients are well-documented;1-3 however, the impact of prophylaxis on mortality is still discussed. Although some meta-analyses have shown a reduction of this indicator,4,5 individually, most studies did not detect significant decreases.1,3 Due to the few benefits in reducing mortality and also due to the threat of developing bacterial resistance, studies and guidelines advise the use of prophylaxis only in high-risk patients, defined by the severity and/or duration of neutropenia.1,6 Other studies even discourage its use, particularly in centers where there is high incidence of resistant bacteria.7−9
Considering that changes in local bacterial epidemiology can impact treatment effectiveness and patient safety, this study assessed whether there was emergence of quinolone resistance related to the increased use of levofloxacin in one HSCT service. Possible changes in the epidemiology of bacteria associated with healthcare-associated infection (HCAI) after the introduction of clinical prophylaxis with levofloxacin were also investigated.
MethodsThe HSCT Service of the Hospital das Clínicas of the Universidade Federal do Paraná (HC/UFPR) adopted prophylaxis with levofloxacin for neutropenic patients in the middle of 2009. An oral dose of 500mg is used once daily from the initial conditioning or the infusion of hematopoietic stem cells until granulocyte recovery or the development of fever and early empirical antimicrobial therapy.10
Patients admitted from January of 2007 to December of 2011 were studied. The period from January of 2007 to December of 2008 was the period preceding the use of prophylactic levofloxacin (period A), and the period from January of 2010 to December of 2011 was characterized by the prophylactic use of levofloxacin (period B). The period from January to December of 2009 was not evaluated to avoid the possibility of bias in the results, since the transition of the protocol occurred in the month of July. Regarding readmissions, if a patient was hospitalized during both of the studied periods, the characteristics of this patient and culture results were considered separately for each period.
The susceptibility of the etiologic agents isolated in cultures and the epidemiology of bacteria linked to HCAI were evaluated in both periods. The frequency of resistance to quinolones was evaluated by microbiological investigations; with the preparation of bacterial susceptibility profiles for periods A and B. Cultures of clinical samples with positive results obtained from hospitalized patients were analyzed. The results considered for the sensitivity profile were: resistant, intermediate, and sensitive. For patients with two diagnostic cultures, different strains for the same etiologic agent were considered when the interval between collections was longer than one month or when there was a change in sensitivity/resistance to at least one antimicrobial agent tested independent of the interval between collections. The use of levofloxacin, cefepime, and meropenem was assessed by defined daily dose (DDD) per 1,000 patient-days.
The Kirby-Bauer method, performed by the diagnostic support unit of the bacteriology department of in HC/UFPR, was used to evaluate the susceptibility profile, in accordance with the recommendations of the National Committee for Clinical and Laboratory Standardization.
Monthly routine epidemiological surveillance records, provided by the hospital's infection control department, were used for HCAI data. The hospital infection rate, the relationship between confirmed clinical and laboratory HCAI, the frequency of etiological agents, and the topography of infections were analyzed.
The concepts and classification of HCAI followed the criteria of the Centers for Disease Control and Prevention (CDC) and the Brazilian criteria for healthcare-associated infections from 2009. Sepsis by coagulase-negative staphylococci (CNS) was defined when the microorganism was identified in two consecutive blood cultures or when the patient was treated empirically with vancomycin due to the results of a culture.
Statistical analyses were used to evaluate the association between the frequency of resistance in the two periods studied, the frequency of etiologic agents, and the characteristics of the population. The statistical software R was used for analysis, applying Pearson's chi-squared test, Fisher's exact test, and the Poisson test. p-values<0.05 were considered to be statistically significant. The analysis was performed by the UFPR statistics laboratory.
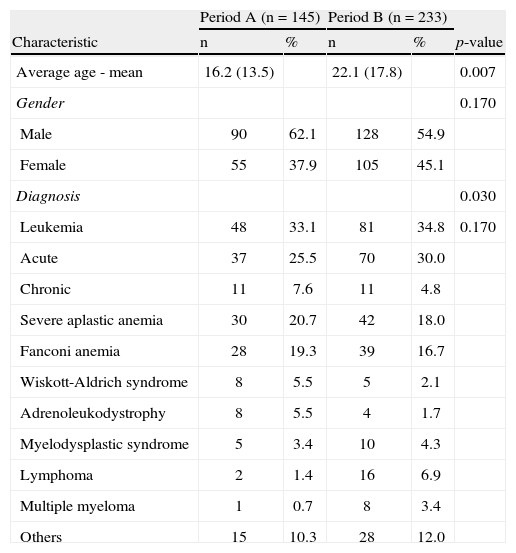
ResultsPatients’ characteristicsA total of 378 patients were analyzed during the study period; 145 were hospitalized in period A and 233 were hospitalized in period B. Table 1 describes the characteristics of patients admitted during both periods.
Characteristics of patients hospitalized during periods A and B.
| Characteristic | Period A (n=145) | Period B (n=233) | p-value | ||
| n | % | n | % | ||
| Average age - mean | 16.2 (13.5) | 22.1 (17.8) | 0.007 | ||
| Gender | 0.170 | ||||
| Male | 90 | 62.1 | 128 | 54.9 | |
| Female | 55 | 37.9 | 105 | 45.1 | |
| Diagnosis | 0.030 | ||||
| Leukemia | 48 | 33.1 | 81 | 34.8 | 0.170 |
| Acute | 37 | 25.5 | 70 | 30.0 | |
| Chronic | 11 | 7.6 | 11 | 4.8 | |
| Severe aplastic anemia | 30 | 20.7 | 42 | 18.0 | |
| Fanconi anemia | 28 | 19.3 | 39 | 16.7 | |
| Wiskott-Aldrich syndrome | 8 | 5.5 | 5 | 2.1 | |
| Adrenoleukodystrophy | 8 | 5.5 | 4 | 1.7 | |
| Myelodysplastic syndrome | 5 | 3.4 | 10 | 4.3 | |
| Lymphoma | 2 | 1.4 | 16 | 6.9 | |
| Multiple myeloma | 1 | 0.7 | 8 | 3.4 | |
| Others | 15 | 10.3 | 28 | 12.0 | |
The average ages were 16.24years and 22.13 (p-value=0.007), the percentages of men were 62.1% and 54.9% (p-value=0.170), and the numbers of HSCTs performed were 161 and 244, for periods A and B, respectively. The most prevalent diagnoses in both periods were leukemia, severe aplastic anemia, and Fanconi anemia. The two groups were evenly distributed with respect to gender, but the distributions regarding age and type of diagnosis (p-value=0.030) were statistically different. Analyzing separately the frequency of leukemia, the commonest diagnosis in both groups, no significant difference was observed (p-value=0.170).
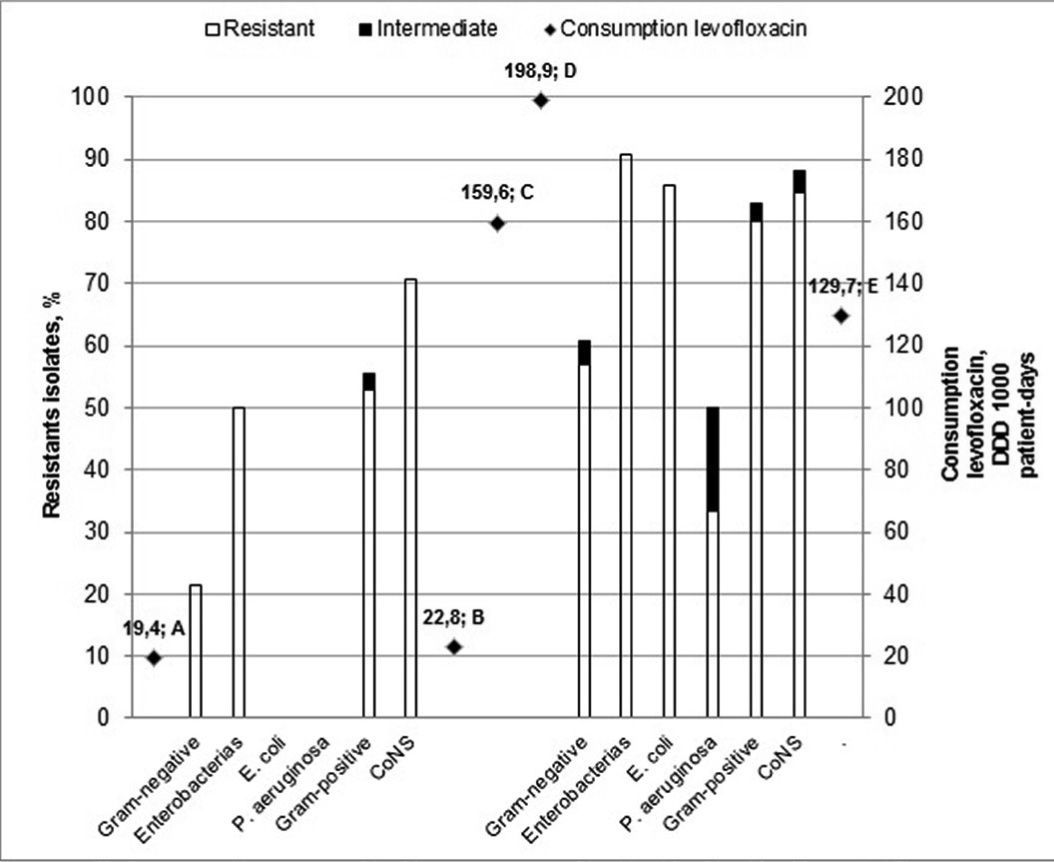
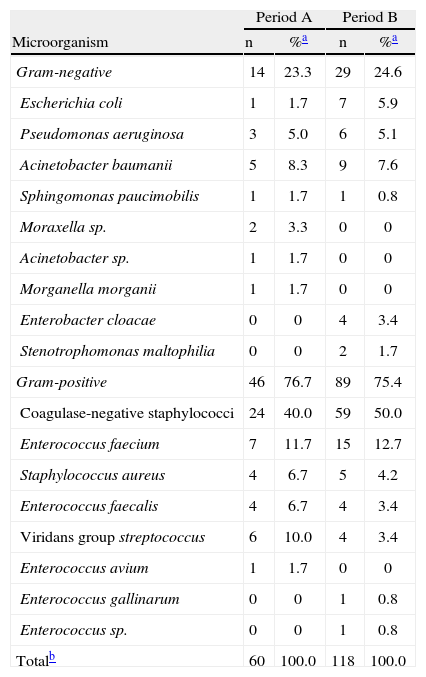
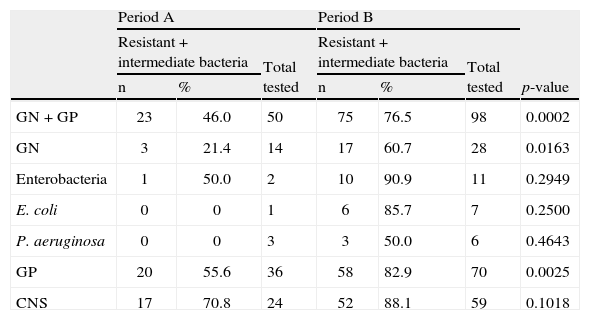
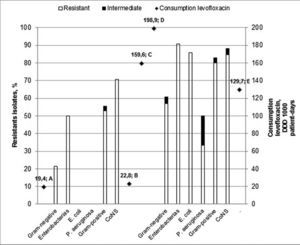
Bacterial susceptibility and antibiotics useIn relation to the susceptibility profile, a total of 178 cultures were analyzed; 60 were isolated in period A and 118 were isolated in period B. The distribution of bacteria isolated in the HSCT service is shown in Table 2. The sensitivity to quinolones was tested in 50 and 98 cultures in periods A and B, respectively. In the analysis of the sensitivity profile, microorganisms with intermediate sensitivity were considered resistant; in the clinical practice, the same conduct is generally used for both intermediate and resistant results. One bacterium with intermediate sensitivity was isolated in period A and three in period B. Table 3 shows the frequency of bacterial resistance into the two periods. The correlation between bacterial resistance to quinolones and the use of levofloxacin is shown in Figure 1. There was a significant increase in resistance in period B compared to period A for all the different types of bacteria isolated (from 46.0% to 76.5%; p-value=0.0002), for Gram-negative bacteria (GN; from 21.4% to 60.7%; p-value=0.0163) and Gram-positive bacteria (GP; from 55.6% to 82.9%; p-value=0.0025). For Enterobacteriaceae, E. coli, P. aeruginosa, and CNS, the increased resistance was not statistically significant.
Distribution of microorganisms used to elaborate the bacterial susceptibility profile isolated from different samples in the Hematopoietic Stem Cell Transplant Service in Periods A and B.
| Microorganism | Period A | Period B | ||
| n | %a | n | %a | |
| Gram-negative | 14 | 23.3 | 29 | 24.6 |
| Escherichia coli | 1 | 1.7 | 7 | 5.9 |
| Pseudomonas aeruginosa | 3 | 5.0 | 6 | 5.1 |
| Acinetobacter baumanii | 5 | 8.3 | 9 | 7.6 |
| Sphingomonas paucimobilis | 1 | 1.7 | 1 | 0.8 |
| Moraxella sp. | 2 | 3.3 | 0 | 0 |
| Acinetobacter sp. | 1 | 1.7 | 0 | 0 |
| Morganella morganii | 1 | 1.7 | 0 | 0 |
| Enterobacter cloacae | 0 | 0 | 4 | 3.4 |
| Stenotrophomonas maltophilia | 0 | 0 | 2 | 1.7 |
| Gram-positive | 46 | 76.7 | 89 | 75.4 |
| Coagulase-negative staphylococci | 24 | 40.0 | 59 | 50.0 |
| Enterococcus faecium | 7 | 11.7 | 15 | 12.7 |
| Staphylococcus aureus | 4 | 6.7 | 5 | 4.2 |
| Enterococcus faecalis | 4 | 6.7 | 4 | 3.4 |
| Viridans group streptococcus | 6 | 10.0 | 4 | 3.4 |
| Enterococcus avium | 1 | 1.7 | 0 | 0 |
| Enterococcus gallinarum | 0 | 0 | 1 | 0.8 |
| Enterococcus sp. | 0 | 0 | 1 | 0.8 |
| Totalb | 60 | 100.0 | 118 | 100.0 |
Susceptibility of isolated bacteria in the Hematopoietic Stem Cell Transplant Service in periods A and B.
| Period A | Period B | p-value | |||||
| Resistant+intermediate bacteria | Total tested | Resistant+intermediate bacteria | Total tested | ||||
| n | % | n | % | ||||
| GN+GP | 23 | 46.0 | 50 | 75 | 76.5 | 98 | 0.0002 |
| GN | 3 | 21.4 | 14 | 17 | 60.7 | 28 | 0.0163 |
| Enterobacteria | 1 | 50.0 | 2 | 10 | 90.9 | 11 | 0.2949 |
| E. coli | 0 | 0 | 1 | 6 | 85.7 | 7 | 0.2500 |
| P. aeruginosa | 0 | 0 | 3 | 3 | 50.0 | 6 | 0.4643 |
| GP | 20 | 55.6 | 36 | 58 | 82.9 | 70 | 0.0025 |
| CNS | 17 | 70.8 | 24 | 52 | 88.1 | 59 | 0.1018 |
GN: Gram-negative; GP: Gram-positive; CNS: coagulase-negative staphylococci.
Considering the use of antibiotics in the period, there was an increase in the use of levofloxacin from 19.44 DDD per 1,000 patient-days in period A to 166.64 in period B. Regarding the systemic use of antibiotics used to treat in presence of clinical evidence of infection, there was an increase in meropenem from 4.59 to 5.33 DDD per 1,000 patient-days and a slight decrease in cefepime from 3.75 to 3.32 DDD per 1,000 patient-days.
Epidemiology of health care-associated infectionA total of 297 infections were recorded in period A and 456 infections in period B; the rates of HCAI were 55.7% and 63.9%, respectively. The most frequent infections in both periods were sepsis and pneumonia (Table 4). Of the total infections, 124 were confirmed by laboratory tests in period A; 69 (55.6%) were caused by bacteria, 45 (36.3%) were caused by viruses, and ten (8.1%) were caused by fungi. In period B, 147 infections were confirmed; 76 (51.7%) were caused by bacteria, 47 (32.0%) were caused by viruses, and 24 (16.3%) were caused by fungi.
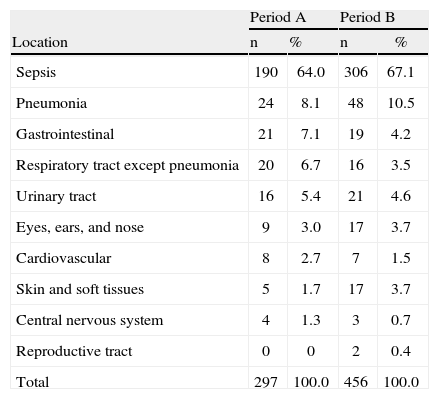
Distribution of healthcare-associated infections by location in periods A and B.
| Location | Period A | Period B | ||
| n | % | n | % | |
| Sepsis | 190 | 64.0 | 306 | 67.1 |
| Pneumonia | 24 | 8.1 | 48 | 10.5 |
| Gastrointestinal | 21 | 7.1 | 19 | 4.2 |
| Respiratory tract except pneumonia | 20 | 6.7 | 16 | 3.5 |
| Urinary tract | 16 | 5.4 | 21 | 4.6 |
| Eyes, ears, and nose | 9 | 3.0 | 17 | 3.7 |
| Cardiovascular | 8 | 2.7 | 7 | 1.5 |
| Skin and soft tissues | 5 | 1.7 | 17 | 3.7 |
| Central nervous system | 4 | 1.3 | 3 | 0.7 |
| Reproductive tract | 0 | 0 | 2 | 0.4 |
| Total | 297 | 100.0 | 456 | 100.0 |
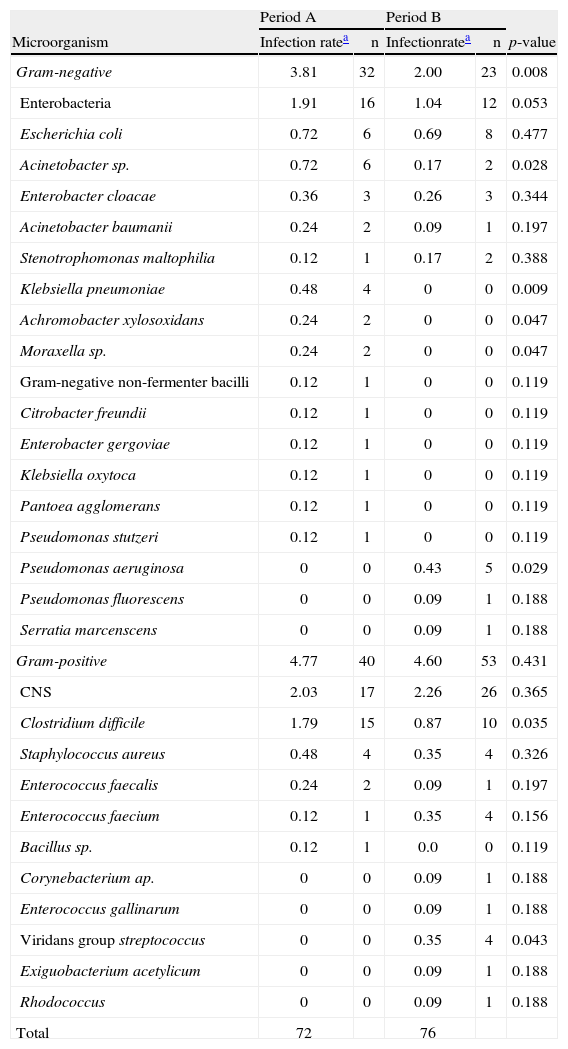
The distribution of isolated bacteria associated with infections is shown in Table 5. A decrease in the rate of GN bacterial infections was observed in period B when compared to period A (3.81 vs. 2.00 cultures/1,000 patient-days; p-value=0.008). The rate of infection by Enterobacteriaceae did not change significantly between periods A and B (1.91 vs. 1.04 cultures/1,000 patient-days; p-value=0.053). Decreases in the rates of Acinetobacter sp. and Klebsiella pneumoniae contributed significantly to reducing the rate of GN infection. In contrast, there was an increase in the rate of infection by Pseudomonas aeruginosa in period B. The rate of infection by GP bacteria was similar in both study periods (4.77 vs. 4.60 cultures/1,000 patient-days; p-value=0.431). The rate of Clostridium difficile decreased in period B (1.79 vs. 0.87 cultures/1,000 patient-days; p-value=0.035) and the rate of Streptococcus viridans increased (0.0 vs. 0.35 cultures/1,000 patients-day; p-value=0.043).
Distribution of microorganisms linked to health care-associated infections during periods A and B.
| Microorganism | Period A | Period B | p-value | ||
| Infection ratea | n | Infectionratea | n | ||
| Gram-negative | 3.81 | 32 | 2.00 | 23 | 0.008 |
| Enterobacteria | 1.91 | 16 | 1.04 | 12 | 0.053 |
| Escherichia coli | 0.72 | 6 | 0.69 | 8 | 0.477 |
| Acinetobacter sp. | 0.72 | 6 | 0.17 | 2 | 0.028 |
| Enterobacter cloacae | 0.36 | 3 | 0.26 | 3 | 0.344 |
| Acinetobacter baumanii | 0.24 | 2 | 0.09 | 1 | 0.197 |
| Stenotrophomonas maltophilia | 0.12 | 1 | 0.17 | 2 | 0.388 |
| Klebsiella pneumoniae | 0.48 | 4 | 0 | 0 | 0.009 |
| Achromobacter xylosoxidans | 0.24 | 2 | 0 | 0 | 0.047 |
| Moraxella sp. | 0.24 | 2 | 0 | 0 | 0.047 |
| Gram-negative non-fermenter bacilli | 0.12 | 1 | 0 | 0 | 0.119 |
| Citrobacter freundii | 0.12 | 1 | 0 | 0 | 0.119 |
| Enterobacter gergoviae | 0.12 | 1 | 0 | 0 | 0.119 |
| Klebsiella oxytoca | 0.12 | 1 | 0 | 0 | 0.119 |
| Pantoea agglomerans | 0.12 | 1 | 0 | 0 | 0.119 |
| Pseudomonas stutzeri | 0.12 | 1 | 0 | 0 | 0.119 |
| Pseudomonas aeruginosa | 0 | 0 | 0.43 | 5 | 0.029 |
| Pseudomonas fluorescens | 0 | 0 | 0.09 | 1 | 0.188 |
| Serratia marcenscens | 0 | 0 | 0.09 | 1 | 0.188 |
| Gram-positive | 4.77 | 40 | 4.60 | 53 | 0.431 |
| CNS | 2.03 | 17 | 2.26 | 26 | 0.365 |
| Clostridium difficile | 1.79 | 15 | 0.87 | 10 | 0.035 |
| Staphylococcus aureus | 0.48 | 4 | 0.35 | 4 | 0.326 |
| Enterococcus faecalis | 0.24 | 2 | 0.09 | 1 | 0.197 |
| Enterococcus faecium | 0.12 | 1 | 0.35 | 4 | 0.156 |
| Bacillus sp. | 0.12 | 1 | 0.0 | 0 | 0.119 |
| Corynebacterium ap. | 0 | 0 | 0.09 | 1 | 0.188 |
| Enterococcus gallinarum | 0 | 0 | 0.09 | 1 | 0.188 |
| Viridans group streptococcus | 0 | 0 | 0.35 | 4 | 0.043 |
| Exiguobacterium acetylicum | 0 | 0 | 0.09 | 1 | 0.188 |
| Rhodococcus | 0 | 0 | 0.09 | 1 | 0.188 |
| Total | 72 | 76 | |||
CNS: coagulase-negative staphylococci.
Fluoroquinolones began to be used as prophylaxis for neutropenic patients in 1980. Two decades after this class of antimicrobials was introduced in cancer treatment centers, several studies demonstrated changes in the epidemiology of etiologic agents.9,11,12 Moreover, changes in the susceptibility profiles of these agents to quinolones have been associated with resistance to other classes of drugs.
The sensitivity profile was prepared to assess bacterial susceptibility (Table 3) in the HSCT service. Data evaluated included results from different biological samples. The bacteria isolated from this set of cultures were not necessarily associated with infections (Table 2). The epidemiology of etiologic agents will be discussed only for bacteria linked to HCAI due to their greater clinical relevance.
An increase in resistance to quinolones from 46.0% to 76.5% (p-value=0.0002) was observed when considering all the bacteria tested from the HSCT service. A retrospective study evaluating 258 patients using prophylaxis with levofloxacin combined with penicillin and doxycycline in a HSCT center demonstrated that there was an increase of 60% in resistance to fluoroquinolone.13
Another study in Italy with 269 patients from 2001 to 2009 associated the use of levofloxacin with the emergence of resistance among GP bacteria; only 13% were sensitive to quinolones.14 In the present study, the frequency of sensitive GP bacteria in the period when the use of levofloxacin increased was 17.1%.
Historically, the group of Enterobacteria, with the pathophysiological mechanism of the translocation of bacteria from gastrointestinal treatment, is one of the greatest causes of bacteremia in neutropenic patients.2,9 In the present study, resistance did not increase significantly for this group. A larger discrepancy was observed in the susceptibility study by Saito et al., who demonstrated a decrease in the resistance of Enterobacteria isolated in blood cultures (from 75% to 17%; p-value=0.0078) when the use of levofloxacin was restricted. The authors concluded that restrictions on the use of prophylaxis with levofloxacin reduce the frequency of resistance.1,2
Studies demonstrate that variations in resistance to quinolones are related to both the amount of quinolones used in the same institution and the frequency of use at different institutions. In a prospective study with 823 patients and 148 isolated bacteria conducted over 16 months, E. coli was resistant to quinolones in 96.5% of patients who received prophylaxis and 44.4% in the group that did not.11 Another study conducted in two different cancer treatment centers in university hospitals, published in 2003 by Kern et al., reported a 64% to 79% proportion of E. coli fluoroquinolone-resistant strains isolated from blood cultures in a hospital with widespread use of prophylactic ciprofloxacin, whereas in a hospital where the use of quinolones was lower, the rate ranged from 0 to 10%.15
In the current study, increased resistance to all the evaluated bacteria was found related to the increased use of levofloxacin in the service (Figure 1). This increase in resistance was statistically significant when analyzing all bacteria (GP and GN) and groups of GP and GN bacteria separately (Table 3). When analyzed separately, the increase in the resistance for the group of Enterobacteria, Pseudomonas aeruginosa, E. coli, and CNS was not statistically significant, but the percentage increase in resistance can demonstrate a trend that should be acknowledged.
In addition, some studies have shown that resistance to quinolones is associated with cross-resistance to other classes of antibiotics.16,17 Among the mechanisms of resistance, mutations in the topoisomerase enzyme and DNA gyrase, transmission of multidrug-resistant genes by plasmids, and reduction of bacterial permeability have been described.17,18
Hopkins et al. reviewed the mechanisms of E. coli and Salmonella sp. resistance. Decreased fluoroquinolone uptake is caused by a decrease in bacterial permeability or over expression of efflux pumps. This resistance is the consequence of a multiple antimicrobial resistance (MAR) phenotype, and has been associated with cross-resistance to many structurally-unrelated antimicrobials, such as beta-lactams, tetracyclines, nalidixic acid, and chloramphenicol. This MAR phenotype is also induced by other antimicrobials, such as tetracyclines, chloramphenicol, salicylates, and many other substances containing phenolic rings that reduce MAR repression.18
Resistance transmission can occur through the expression of a gene responsible for plasmid-mediated quinolone resistance (PMQR). It is known that this gene encodes a protein that protects DNA gyrase inhibition by fluoroquinolones. Hopkins et al. reported that the presence of the plasmid PMG252 caused a low basal level of resistance in E. coli. However, when added to the other mutations in the DNA gyrase enzyme, mutations resulting in decreased permeability and increased bacterial expression of efflux pumps promoted the selection of higher resistance. Furthermore, it was demonstrated that the plasmid PMG252 also confers resistance to aztreonam, ceftazidime, cefotaxime, cefoxitin, cefotetan, chloramphenicol, kanamycin, gentamicin, streptomycin, sulfafurazole, tobramycin, and trimethoprim.18
There are reports that the cross resistance to other structurally-unrelated antimicrobials may be related to the increase in multidrug resistant bacteria. In a retrospective study that analyzed 622 bacteremias in 2005 and 2006, prophylaxis with fluoroquinolone was compared with prophylaxis using other drugs and the absence of prophylaxis. Prophylaxis with quinolones was strongly associated with the isolation of methicillin-resistant S. aureus, as well as being associated with the emergence of multidrug-resistant E. coli and P. aeruginosa.19
Dancer described that quinolones were involved in a resistance mechanism and the increased virulence of S. aureus. The horizontal transfer of genetic elements of resistance and virulence, known as the SOS, could be induced by the use of quinolones. In addition, sub-inhibitory concentrations of quinolones would be involved in induction of adhesins that results in increased adherence of S. aureus to plasma proteins. The increase in host cell intracellular persistence and a high cytotoxic capacity after removal of the antibiotic, related to the recurrence of infections, appears to be associated.20
Epidemiology of healthcare-associated infectionsThe reduction of infections with the use of prophylaxis with fluoroquinolone is documented in the literature.11,12 In a meta-analysis published in 1998, a significant reduction in the number of infections by GN microorganisms, microbiologically documented infections, and in total duration of fever was demonstrated compared to the group that did not use clinical quinolone prophylaxis.21
The present study found a significant increase in the rate of HCAI in period B. The larger number of complex transplants, including unrelated donors where the patient becomes more vulnerable to infections due to the more rigorous immunosuppressive treatment, may be a justification for this finding. Furthermore, differences in baseline characteristics of patients may be a factor that influences clinical severity.
Among the infections confirmed by laboratorial analysis, there was a proportional increase of fungi isolated in cultures. Although the number of laboratory confirmed infections is lower in relation to total HCAI (41.7% in period A and 32.2% in period B), this increase may also be related to the higher number of complex transplants performed. Furthermore, it is important to remember that, during period B, the HSCT unit was rebuilt, a condition that may predispose the environment to an increased dispersion of fungi, such as Aspergillus sp. and Fusarium sp.
Regarding bacteria linked to HCAI (Table 5), as demonstrated in other studies, there was a significant decrease in the frequency of GN bacteria. Saito et al. demonstrated a reduction in bacteremia caused by GN during the period when there was no restriction on the use of fluoroquinolones as prophylaxis (9.6 vs. 3.6%; p-value=0.0008).12 Craig et al. also found a decrease in GN bacteremia in the period when prophylaxis with fluoroquinolones was used, compared to the period when no prophylaxis was used (4.7 vs. 1.8 cultures/1,000 patient-days; p-value<0.05).3
One expected result when using prophylaxis with fluoroquinolone is decreased GN bacterial infections, especially enterobacterial infections. In the present study, there was no significant decrease in the rate of enterobacterial infections. In contrast, a study conducted in Japan reported a significant decrease in the percentage of infection by Enterobacteriaceae on total patients (8.2 vs. 2.0; p-value<0.0001) in the period without restrictions on prophylaxis with fluoroquinolones.12 Furthermore, it is expected that with the use of quinolones there is a decrease in the consumption of broad-spectrum systemic antibiotics, as the use of this class for prophylaxis should decrease the number of episodes of bacterial infection. However, in the present study there was an increase in the consumption of meropenem in period B and a very slight decrease in the consumption of cefepime. Therefore, despite a decrease in the incidence of infections by GN bacteria, there was no decrease in the use of broad-spectrum antibiotics.
A significant increase in the rate of S. viridans infections was observed in the current study (p-value=0.043). Other studies reported that this finding was related to the use of antibiotics at suboptimal minimum inhibitory concentrations followed by translocation of this agent from the gastrointestinal tract to the bloodstream.14 In another study, this event was related to severe mucositis associated with respiratory failure and shock.22
An increased frequency of Clostridium difficile diarrhea has been shown associated with the use of fluoroquinolones.23,24 Quinolones in the gut only have a limited effect on the GN microbiota, causing the proliferation of the intact anaerobic population. Furthermore, the increase of diarrhea caused by C. difficile has been associated with ineffective infection prevention measures.17 A decrease in the proportion of infections caused by C. difficile (p-value=0.035) was observed in this study, which can be explained by the implementation of precautionary contact measures and changes in the surface disinfection process in 2009 by the hospital's infection control team.
Besides the risk of infection and colonization by multi-resistant microorganisms, studies describe decreased effectiveness of prophylaxis with fluoroquinolones in centers where there is high prevalence of resistance to this class of antimicrobial agent.7,25 In a study conducted between 2000 and 2003 to evaluate the usefulness of prophylaxis with fluoroquinolone in neutropenic patients, it was observed that in hospitals with a high frequency of resistance, prophylaxis did not prevent febrile events, nor decrease mortality rates.9 Bow indicated that an analysis of the need to use prophylaxis with fluoroquinolone is important when the resistance is greater than 20%.17 Another study reported a decreased efficacy to reduce GN bacterial infections when the resistance was greater than 30%.26
The aim of this study was to investigate the emergence of quinolone resistance among all bacteria detected and recorded in the period. The emergence of bacterial resistance observed only among bacteria associated with infection was not studied, making it difficult to extrapolate the data on resistance to HCAI. Another limitation is the small number of etiological agents isolated as compared to the total number of infections, decreasing the representation of the results. Furthermore, clinical outcomes, such as infection-related death, an important clinical impact indicator against changes in epidemiology and bacterial susceptibility, were not assessed in this study.
ConclusionThe use of prophylaxis with levofloxacin contributed to a decrease in the GN bacterial infection rate; however, there was no significant decrease in the rate of infection by Enterobacteriaceae and no decrease in the consumption of broad-spectrum antibiotics, which suggests that the effectiveness of the prophylaxis protocol should be reviewed. Considering the risks and benefits of antimicrobial prophylaxis, it is important to note the effect of prophylaxis on the emergence of bacterial resistance, reducing the effectiveness of empiric broad-spectrum antibiotics, and altering the effect of resistance on the clinical outcomes of neutropenic fever. Considering that increased resistance can lead to decreased efficacy of prophylaxis with quinolones, and also that the prophylaxis decreases empirical treatment options, the use of this drug should be reconsidered. It is true that every decision regarding the choice of empiric or prophylactic drugs should be guided by the local epidemiology of each hospital. Therefore, it is necessary to monitor the profile of sensitivity and resistance of bacterial etiologic agents isolated in these centers, in order to guide the selection and/or change of therapeutic protocols.
Conflicts of interestThe authors declare no conflicts of interest.