Chronic graft-versus-host disease is a serious complication of allogeneic hematopoietic cell transplantation, and the mouth is one of the affected sites.
ObjectiveThe aim of this study was to evaluate the oral features of this disease after hematopoietic cell transplantation.
MethodsThis was a cross-sectional multicenter study that enrolled patients submitted to transplantation. Oral evaluations used the National Institutes of Health criteria, salivary flow rates, and the range of mouth opening. Pain and xerostomia were evaluated through a visual analogue scale. Patients were divided into two groups based on the transplantation time (up to one year and more than one year).
ResultsOf the 57 evaluated recipients, 44 had chronic graft-versus-host disease: ten (22.72%) in the group with less than one year after transplantation, and 34 (77.27%) in the group with more than one year after transplantation. Lichenoid/hyperkeratotic plaques, erythematous lesions, xerostomia, and hyposalivation were the most commonly reported oral features. Lichenoid/hyperkeratotic plaques were significantly more common in patients within the first year after the transplant. The labial mucosa was affected more in the first year. No significant changes occurred in the frequency of xerostomia, hyposalivation, and reduced mouth opening regarding time after transplantation.
ConclusionOral chronic graft-versus-host disease lesions were identified early in the course of the disease. The changes observed in salivary gland function and in the range of mouth opening were not correlated with the time after transplantation.
© 2014 Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. All rights reserved.
Allogeneic hematopoietic cell transplantation (HCT) is associated with early and late oral complications. Most of these complications are related to graft-versus-host disease (GVHD) and may cause transitory and/or permanent sequelae. Many studies have reported oral manifestations of chronic GVHD (cGVHD),1-3 but to the authors’ knowledge, only one study has addressed the temporal outcomes of some of these features.4 Most studies focus on specific oral alterations of cGVHD, and have not used the National Institutes of Health (NIH) criteria to evaluate the oral features.
cGVHD affects from 50% to 80% of the adult patients who undergo HCT, regardless of prophylaxis and donor-patient matching techniques.2
Oral manifestations can be the first signs of cGVHD, and might be considered a disease marker.3,5 These oral manifestations are observed in the majority of cases of cGVHD,2,5-7 and are considered an important cause of morbidity and loss of quality of life in long-term survivors.5
According to the NIH Consensus Criteria for GVHD, the disease is currently diagnosed by its clinical manifestations and no longer according to time post-transplantation.8 The clinical manifestations of cGVHD are classified as diagnostic, distinctive, or common clinical features. The finding of a ‘diagnostic’ feature of cGVHD establishes the condition without further testing. In the oral cavity, these features include lichen planus-like lesions, hyperkeratotic plaques, and restriction of mouth opening due to skin sclerosis. ‘Distinctive’ features are likely to support the diagnosis of cGVHD; however, they are not enough to establish the diagnosis. The oral ‘distinctive’ features include xerostomia, mucoceles, mucosal atrophy, pseudomembranes, and ulcers. ‘Common’ features of cGVHD refer to the manifestations found in both acute GVHD and cGVHD, which include gingivitis, mucositis, erythema, and pain.8
Some clinical changes of oral cGVHD such as vasculitis-like features or a telangiectatic appearance of the mucosa, inflammation, and loss of the stippling of the attached gingiva may represent early alterations of oral cGVHD, even though they are not considered in the NIH criteria.2 Erosive lesions are observed in the most severe forms of cGVHD, and are followed by pain, which may interfere in oral hygiene and food ingestion.9 Xerostomia generally causes discomfort, but hyposalivation, that is, reduced salivary flow rates (SFR), which have more serious consequences, have not been included in the NIH criteria.10-12
Temporal features of systemic cGVHD have previously been addressed. However, although oral cGVHD has been analyzed in many publications,1−3 data regarding the temporal relationship of oral features is lacking. The present study aimed to perform a cross-sectional evaluation of the oral features of cGVHD according to the time after HCT.
MethodsThis was a cross-sectional multicenter study conducted in two hospitals in Brazil: Hospital Universitário Clementino Fraga Filho (HUCFF) of the Universidade Federal do Rio de Janeiro (UFRJ) and the Center for Hematology and Hemotherapy of the Universidade Estadual de Campinas, from January of 2008 to January of 2011. Adult patients who underwent HCT for hematological conditions were included. All patients signed an informed consent. This study was approved by the institution’s review board and conducted in accordance with the Helsinki Declaration as revised in 2008.
Demographic and clinical characteristics were assessed from medical records. After the transplantation, oral evaluations were performed by a trained dentist. The oral exam was performed using frontal light-emitting diode illumination, with the patient sitting on a chair.
Oral features were classified according to NIH criteria as diagnostic, distinctive, and common features.8 These features referred to any changes related to oral signs and symptoms (mucosal lesions, perception of changes in salivary flow rate and moisture, changes in sensitivity, and reduction of mouth opening). Oral lesions were defined as morphological changes of the oral mucosa.
The severity of oral symptoms was scored according to the NIH criteria:8 no symptoms (0); mild symptoms with disease signs not limiting the oral intake (1); moderate symptoms with disease signs, with partial limitation of oral intake (2); and severe symptoms with disease signs and major limitation of oral intake (3). Moreover, the patients graded oral sensitivity through a visual analogue scale (VAS) graduated from 0 (no sensitivity) to 10 (worst possible pain).13,14 Positive sensitivity was recorded when patients reported symptoms greater than 0 on the VAS.
Resting SFR were used to assess salivary function under standard conditions.15 Saliva samples were collected between 9:00 a.m and 11:30a.m.16 Participants were asked not to eat until the exam was performed. Patients were instructed to spit the accumulated saliva periodically into a disposable cup for five minutes. They were instructed not to eat nor swallow during the exam.16 The liquid part of the SFR was then measured with a disposable syringe. The SFR results were determined as milliliters per minute, and reduced SFR was defined as<0.3mL/min.11
Oral dryness was also evaluated using a VAS graduated from 0 (no dryness) to 10 (the worst possible oral dryness). To eliminate common causes of dry mouth feeling, xerostomia was considered when the patient recorded a score>2.13
The oral involvement of scleroderma was evaluated using a Willis compass to measure the maximum range of mouth opening (RMO). The device measured the midline distance from the border of the central upper incisors to the border of the central lower incisors. Reduced RMO was defined as<35mm.17
In order to analyze the temporal manifestations of cGVHD, the patients were separated in two groups: within one year post-HCT and over one year post-HCT. In general, cGVHD develops from three to 15 months after HCT,1 and the period of one year post-HCT has been used in some studies to evaluate cGVHD.18,19 The date of the transplant was an accurate datum and was available for all patients.
The SPSS© version 13.0 (IBM - Chicago, USA) was used to store and analyze data. The differences between groups were analyzed with Fisher’s exact test and the Mann–Whitney test for the comparison of categorical and continuous variables, respectively. The level of significance was set as a p-value<0.05.
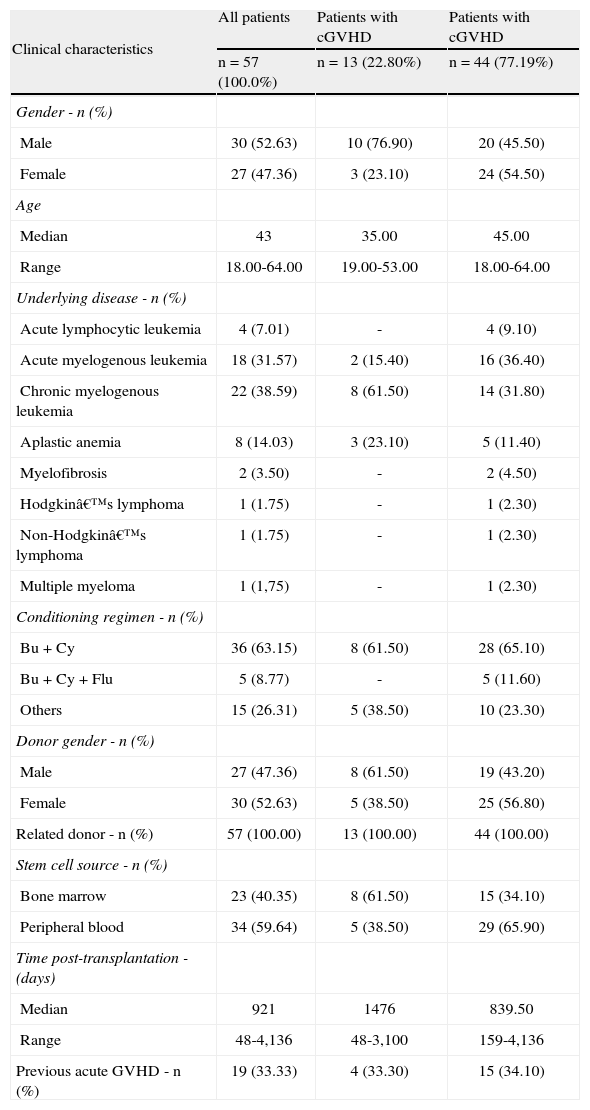
ResultsFifty-seven patients who underwent HCT were analyzed; 27 (47.4%) women and 30 (52.6%) men. The median age was 43 years, ranging from 18 to 64 years. Among the patients submitted to HCT, 68.4% presented oral features. The clinical and demographic characteristics of the 57 HCT patients are shown in Table 1. The oral features of the HCT patients included in the study are listed in Table 2.
Clinical and demographic characteristics of the 57 patients submitted to hematopoietic cell transplantation.
| Clinical characteristics | All patients | Patients with cGVHD | Patients with cGVHD |
| n=57 (100.0%) | n=13 (22.80%) | n=44 (77.19%) | |
| Gender - n (%) | |||
| Male | 30 (52.63) | 10 (76.90) | 20 (45.50) |
| Female | 27 (47.36) | 3 (23.10) | 24 (54.50) |
| Age | |||
| Median | 43 | 35.00 | 45.00 |
| Range | 18.00-64.00 | 19.00-53.00 | 18.00-64.00 |
| Underlying disease - n (%) | |||
| Acute lymphocytic leukemia | 4 (7.01) | - | 4 (9.10) |
| Acute myelogenous leukemia | 18 (31.57) | 2 (15.40) | 16 (36.40) |
| Chronic myelogenous leukemia | 22 (38.59) | 8 (61.50) | 14 (31.80) |
| Aplastic anemia | 8 (14.03) | 3 (23.10) | 5 (11.40) |
| Myelofibrosis | 2 (3.50) | - | 2 (4.50) |
| Hodgkin’s lymphoma | 1 (1.75) | - | 1 (2.30) |
| Non-Hodgkin’s lymphoma | 1 (1.75) | - | 1 (2.30) |
| Multiple myeloma | 1 (1,75) | - | 1 (2.30) |
| Conditioning regimen - n (%) | |||
| Bu+Cy | 36 (63.15) | 8 (61.50) | 28 (65.10) |
| Bu+Cy+Flu | 5 (8.77) | - | 5 (11.60) |
| Others | 15 (26.31) | 5 (38.50) | 10 (23.30) |
| Donor gender - n (%) | |||
| Male | 27 (47.36) | 8 (61.50) | 19 (43.20) |
| Female | 30 (52.63) | 5 (38.50) | 25 (56.80) |
| Related donor - n (%) | 57 (100.00) | 13 (100.00) | 44 (100.00) |
| Stem cell source - n (%) | |||
| Bone marrow | 23 (40.35) | 8 (61.50) | 15 (34.10) |
| Peripheral blood | 34 (59.64) | 5 (38.50) | 29 (65.90) |
| Time post-transplantation - (days) | |||
| Median | 921 | 1476 | 839.50 |
| Range | 48-4,136 | 48-3,100 | 159-4,136 |
| Previous acute GVHD - n (%) | 19 (33.33) | 4 (33.30) | 15 (34.10) |
GVHD: graft-versus-host disease; cGVHD: chronic graft-versus-host disease; Busulfan: Bu; Cyclophosphamide: Cy; Fludarabine: Flu.
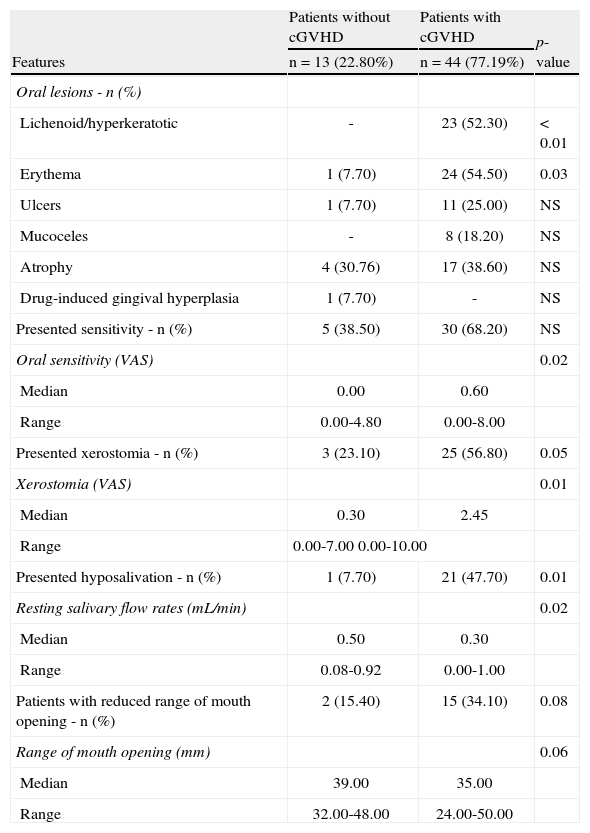
Oral features of the 57 patients submitted to hematopoietic cell transplantation.
| Features | Patients without cGVHD | Patients with cGVHD | p-value |
| n=13 (22.80%) | n=44 (77.19%) | ||
| Oral lesions - n (%) | |||
| Lichenoid/hyperkeratotic | - | 23 (52.30) | < 0.01 |
| Erythema | 1 (7.70) | 24 (54.50) | 0.03 |
| Ulcers | 1 (7.70) | 11 (25.00) | NS |
| Mucoceles | - | 8 (18.20) | NS |
| Atrophy | 4 (30.76) | 17 (38.60) | NS |
| Drug-induced gingival hyperplasia | 1 (7.70) | - | NS |
| Presented sensitivity - n (%) | 5 (38.50) | 30 (68.20) | NS |
| Oral sensitivity (VAS) | 0.02 | ||
| Median | 0.00 | 0.60 | |
| Range | 0.00-4.80 | 0.00-8.00 | |
| Presented xerostomia - n (%) | 3 (23.10) | 25 (56.80) | 0.05 |
| Xerostomia (VAS) | 0.01 | ||
| Median | 0.30 | 2.45 | |
| Range | 0.00-7.00 0.00-10.00 | ||
| Presented hyposalivation - n (%) | 1 (7.70) | 21 (47.70) | 0.01 |
| Resting salivary flow rates (mL/min) | 0.02 | ||
| Median | 0.50 | 0.30 | |
| Range | 0.08-0.92 | 0.00-1.00 | |
| Patients with reduced range of mouth opening - n (%) | 2 (15.40) | 15 (34.10) | 0.08 |
| Range of mouth opening (mm) | 0.06 | ||
| Median | 39.00 | 35.00 | |
| Range | 32.00-48.00 | 24.00-50.00 | |
cGVHD: chronic graft-versus-host disease; HCT: hematopoietic cell transplantation; NS: non-significant; VAS: visual analogue scale.
Forty-four (77.2%) patients developed cGVHD. Among the cGVHD patients, 88.63% presented oral features. This analysis was focused primarily on patients with cGVHD, as the majority of the HCT recipients developed this condition. Among the patients who developed cGVHD, the mouth was the most frequently affected site (88.63%), regardless of the different stem cell sources (bone marrow: 71.4%; peripheral blood: 66.7%). The most affected organs were the mouth, skin, liver, eyes, and lungs.
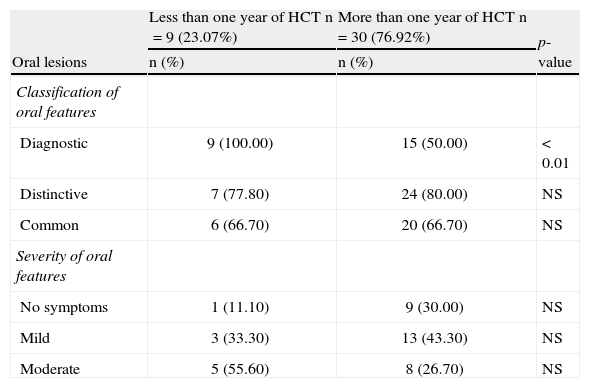
The most common oral features observed in cGVHD patients were oral lesions (70.45%), xerostomia (56.81%), reduced SFR (47.72%), and reduced RMO (31.81%). The most common types of oral lesions in cGVHD patients were erythema (54.54%) and lichenoid/hyperkeratotic lesions (52.27%). Lichenoid lesions are considered a diagnostic feature by the NIH criteria, and were observed in all patients that presented oral features within one year post-HCT; many patients presented more than one type of oral manifestation. The NIH classification and grading of the cGVHD patients who presented oral features are listed in Table 3. Data on the characteristics of the oral lesions, salivary function, and RMO according to the time post-transplantation are listed in Tables 4, 5, and 6, respectively.
Classification and grading of oral features of chronic graft-versus-host disease according to the National Institutes of Health criteria (n=39).
| Oral lesions | Less than one year of HCT n=9 (23.07%) | More than one year of HCT n=30 (76.92%) | p-value |
| n (%) | n (%) | ||
| Classification of oral features | |||
| Diagnostic | 9 (100.00) | 15 (50.00) | < 0.01 |
| Distinctive | 7 (77.80) | 24 (80.00) | NS |
| Common | 6 (66.70) | 20 (66.70) | NS |
| Severity of oral features | |||
| No symptoms | 1 (11.10) | 9 (30.00) | NS |
| Mild | 3 (33.30) | 13 (43.30) | NS |
| Moderate | 5 (55.60) | 8 (26.70) | NS |
Some patients presented more than one kind of oral feature and in different oral sites.
HCT: hematopoietic cell transplantation; NS: non-significant.
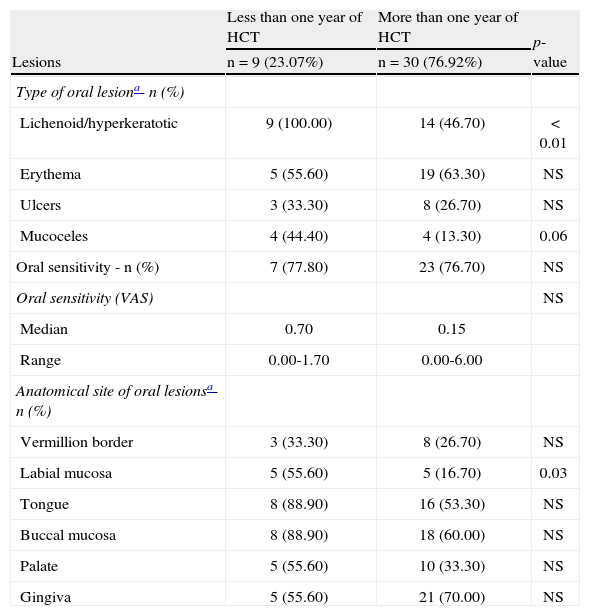
Early and late characteristics of oral lesions in chronic graft-versus-host disease (n=39).
| Lesions | Less than one year of HCT | More than one year of HCT | p-value |
| n=9 (23.07%) | n=30 (76.92%) | ||
| Type of oral lesiona- n (%) | |||
| Lichenoid/hyperkeratotic | 9 (100.00) | 14 (46.70) | < 0.01 |
| Erythema | 5 (55.60) | 19 (63.30) | NS |
| Ulcers | 3 (33.30) | 8 (26.70) | NS |
| Mucoceles | 4 (44.40) | 4 (13.30) | 0.06 |
| Oral sensitivity - n (%) | 7 (77.80) | 23 (76.70) | NS |
| Oral sensitivity (VAS) | NS | ||
| Median | 0.70 | 0.15 | |
| Range | 0.00-1.70 | 0.00-6.00 | |
| Anatomical site of oral lesionsa- n (%) | |||
| Vermillion border | 3 (33.30) | 8 (26.70) | NS |
| Labial mucosa | 5 (55.60) | 5 (16.70) | 0.03 |
| Tongue | 8 (88.90) | 16 (53.30) | NS |
| Buccal mucosa | 8 (88.90) | 18 (60.00) | NS |
| Palate | 5 (55.60) | 10 (33.30) | NS |
| Gingiva | 5 (55.60) | 21 (70.00) | NS |
HCT: hematopoietic cell transplant; NS: non-significant; VAS: visual analogue scale.
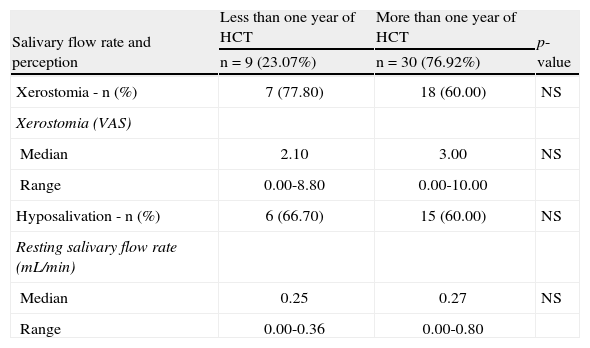
Early and late characteristics of salivary function in chronic graft-versus-host (n=39).
| Salivary flow rate and perception | Less than one year of HCT | More than one year of HCT | p-value |
| n=9 (23.07%) | n=30 (76.92%) | ||
| Xerostomia - n (%) | 7 (77.80) | 18 (60.00) | NS |
| Xerostomia (VAS) | |||
| Median | 2.10 | 3.00 | NS |
| Range | 0.00-8.80 | 0.00-10.00 | |
| Hyposalivation - n (%) | 6 (66.70) | 15 (60.00) | NS |
| Resting salivary flow rate (mL/min) | |||
| Median | 0.25 | 0.27 | NS |
| Range | 0.00-0.36 | 0.00-0.80 |
HCT: hematopoietic cell transplant; VAS: visual analogue scale; NS: non-significant.
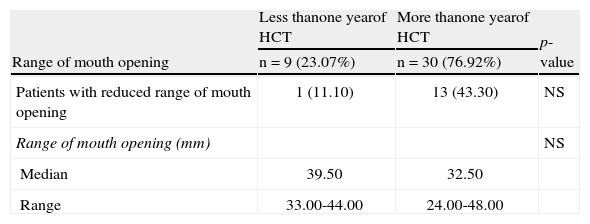
Early and late characteristics of the range of mouth opening in chronic graft-versus-host disease (n=39).
| Range of mouth opening | Less thanone yearof HCT | More thanone yearof HCT | p-value |
| n=9 (23.07%) | n=30 (76.92%) | ||
| Patients with reduced range of mouth opening | 1 (11.10) | 13 (43.30) | NS |
| Range of mouth opening (mm) | NS | ||
| Median | 39.50 | 32.50 | |
| Range | 33.00-44.00 | 24.00-48.00 |
HCT: hematopoietic cell transplant; NS: non-significant.
There were no differences related to the time of transplantation regarding the features of xerostomia, SFR, or RMO. Moreover, there was no relationship between hyposalivation and gender (p-value=0.39) or age (p-value=0.93). Among the patients who had cGVHD, 54.5% presented concomitant xerostomia and sensitivity. Only ten (22.7%) patients had concomitant ulcers and sensitivity.
DiscussionThis study was relevant due to the evaluation of all oral cGVHD features in a standardized manner. Most of the studies on oral cGVHD evaluated isolated aspects of the disease. Moreover, the present study was one of the first to evaluate these features using the NIH criteria. Besides the subjective way that the NIH defines these criteria, objective evaluations were performed for the signs and symptoms of oral cGVHD. To the best of the authors’ knowledge, there is no study correlating the frequency and the severity of oral cGVHD lesions with the time post-HCT. Only one study evaluated the temporal correlation of salivary flow and taste changes with time post-HCT, but the authors also included patients without GVHD in the analysis.4
In the present study, the frequency of cGVHD was consistent with data reported in the literature, even when the majority received GVHD prophylaxis to prevent the disease.9 Among cGVHD patients, the mouth was the most affected site, regardless of the time post-HCT. When tracking oral aspects of cGVHD patients, it is observed that the morphological changes of the existing alterations vary along the course of the disease. Considering the temporal differences in oral manifestations of cGVHD, the patients in this study were divided into two groups; more than and less than one year after HCT.
Diagnostic features were significantly more frequent in cGVHD patients in the less than one year group; the labial mucosa was more affected in the first year after transplantation. In the literature, lichenoid lesions, considered a diagnostic feature, have been observed in over 80% of cGVHD patients.1 In the present study, nearly half of the cGVHD patients presented lichenoid oral lesions/hyperkeratotic plaques. However, when the temporal factor was considered, cGVHD patients with less than one year post-HCT presented significantly more lichenoid lesions/hyperkeratotic plaques. This finding suggests the presence of time-related changes regarding this type of oral lesion. Healthcare professionals should be aware of these lesions as the first oral sign of cGVHD.
Oral pain has been reported as a frequent symptom in cGVHD,20 and is generally associated with ulcerations.14 In fact, the majority of the cGVHD patients in the present study presented mild to moderate symptoms. However, only a few patients presented sensitivity related to the oral ulcers. Moreover, there were no significant differences in the oral symptomatology of cGVHD patients in relation to the time post-HCT. No significant temporal differences were observed for the other oral signs or symptoms.
Reduced SFR is related to several systemic conditions, such as Sjögren’s syndrome, diabetes mellitus, hepatitis C, and some immunological diseases, as well as some medications.10 After transplantation, late alterations in salivary function are related to cGVHD.12,21,22 Severe involvement of the salivary glands causes permanent destruction of the salivary parenchyma, and oral dryness may be observed.2,21,22 In cGVHD, there is a progressive reduction in the production of saliva due to parenchymal atrophy of the minor salivary glands.12,21,22 Apparently, this progressive salivary dysfunction was not observed in the cGVHD population of the present study, as there were no significant temporal differences observed in SFR. The understanding of this finding should be a focus of future studies.
Although older patients are more likely to have hyposalivation, the most likely causes are medications and disease.23 In the studied population, there were no significant differences between the reduced SFR and age.
The feeling of dry mouth does not necessarily correlate to signs of hyposalivation.12 In the present study, 9.1% of cGVHD patients with xerostomia presented normal SFR. This may be explained by the changes in the sialochemistry or by sensory alterations.24
There was no correlation between oral mucosa changes and salivary dysfunction in the patients with oral cGVHD of the present study. This is in agreement with a study that reported that salivary gland and oral mucosa involvement are separate features of cGVHD.12
Scleroderma is a rare and late manifestation of cGVHD.25 Sclerodermatous plaques on the lips and in the oral tissues may result in the impairment of joint mobility, leading to a permanent restriction of mouth opening.26,27 In the present study, there were no significant differences in the RMO of the cGVHD patients in relation to the time post-HCT.
The frequency of oral alterations in patients who received HCTs was high, even among those who did not develop cGVHD (61.5%). This figure is higher than the prevalence of oral alterations in the Brazilian population (22.0%).28 The oral alterations observed in HCT recipients without cGVHD may be associated with the treatment for the hematologic condition, such as cyclosporine-related gingival hyperplasia.29 The oral erythema and ulceration observed in these patients may result from injuries that frequently occur in the oral mucosa. The four cases of atrophy in patients with no history of cGVHD may represent early cases of the disease. Among these four patients, only one had less than one year post-transplantation; in this case, acute GVHD was considered in the differential diagnosis. Oral sensitivity was significantly higher in patients with cGVHD, which may be explained by the fact that cGVHD patients have more oral features. Xerostomia and hyposalivation were also significantly higher in patients who developed cGVHD than in other HCT recipients; the mean SFR of HCT recipients who did not develop cGVHD was considered to be within the normal limits.30 Among the patients who did not develop GVHD, there was only one who presented hyposalivation and three who presented xerostomia. This may be due to the fact that early salivary gland dysfunction in post-HCT may be related to toxicity of the conditioning regimen.11,13,15
One of the limitations of this study was its sample size. Although the study was performed in two hematological centers, there were still difficulties in recruiting patients due to the fact that, currently, few patients receive HCT. The cross-sectional analysis may also present some limitation, especially because the majority of patients had more than one year of post-HCT. Future studies should prospectively evaluate the oral features of cGVHD, correlating them to features in other organs.
ConclusionThe frequency of oral changes in patients who received HCT was high, even for those who did not develop cGVHD. Among the patients with cGVHD, the oral diagnostic features of lichenoid/hyperkeratotic lesions were more frequently found in patients in the early stage of the disease. There were no statistically significant differences in the frequency of oral mucosal sensitivity, xerostomia, hyposalivation, and RMO in cGVHD patients with less than one year of transplantation when compared to those with more than one year post-HCT.
Conflicts of interestThe authors declare no conflicts of interest.
This research was supported by the Conselho Nacional de Desenvolvimento Científico (CNPq: #472817/2007-8) and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ: #E-26/170.794/2007 grant to S. R. Torres)