Hypogonadism is one of the most frequent complications in transfusion-dependent thalassemia patients and early recognition and treatment is the core element in restoring impaired gonadal function. Despite the high burden of disease, relevant studies are scarcely addressing the gonadal function of such patients in Bangladesh. The pattern of gonadal function in transfusion-dependent thalassemia patients must be characterized before planning a generalized management plan. Moreover, since iron overload is a key reason behind hypogonadism in thalassemia patients, investigating the role of serum ferritin level as a diagnostic tool for hypongadism was also an aim of this study.
MethodsThis cross-sectional study was conducted at the Department of Transfusion Medicine of the Bangabandhu Sheikh Mujib Medical University. According to the inclusion and exclusion criteria, a total of 94 patients were enrolled in this study. A detailed history and thorough clinical examination were carried out in each patient and recorded using a pretested structured questionnaire. In addition, the laboratory assessment of serum ferritin, luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone and estradiol in serum were also performed. The data were analyzed using the STATA (v.16).
ResultsThe mean age of the patients with transfusion-dependent thalassemia was 18.81 ± 4.65 (SD), with 53.3% of the patients being male. The overall prevalence of hypogonadism was 35.11%, 18.1% being normogonadotropic, 11.7% being hypogonadotropic and 5.3% being hypergonadotropic. The serum ferritin level was significantly higher (p < 0.001) in patients with hypogonadism (Eugonadal: 2,174.79 (± 749.12) ng/ml; Hypogonadal: 3,572.59 (± 1,199.49) ng/ml). The area under the receiver operating characteristic (ROC) curve of serum ferritin was high (0.83) and the p-value was highly significant (< 0.001).
ConclusionTherefore, the serum ferritin level and gonadal hormone analysis of transfusion-dependent thalassemia patients can be considered a screening tool for assessing gonadal function and early detection and prevention of hypogonadism.
Thalassemia is one of the most common genetic hemoglobinopathies that can result in severe anemia.1,2 It is highly prevalent in Southeast Asia, the Indian subcontinent and Mediterranean and Middle Eastern countries, collectively known as the ‘World Thalassemia Belt’. In Bangladesh, 6 to 12% of the population are carriers of a gene causing thalassemia.3 Thalassemia is characterized by the partial or complete deficiency in the synthesis of α or β-globin chains that compose the major adult hemoglobin (α2β2). Patients with an absolute deficiency of the α or β-globin chains require lifelong blood transfusion and are denoted as the transfusion-dependent thalassemia group.4 With regular transfusion, the life expectancy and survival rate of thalassemia patients dramatically improved from the first to fifth decades of life.5 However, it leads to iron overload, which is accumulated within different tissues, including endocrine glands, resulting in a functional imbalance, among which gonadal dysfunction is the most common.6,7 In addition to the iron overload, factors, such as ferritin level, genotype, transfusion frequency, starting age and iron chelation efficiency, also play a significant role.8 Hypogonadotropic hypogonadism, or secondary hypogonadism resulting from iron deposition in the pituitary gonadotrope, is more commonly found, whereas gonadal iron deposition in ovaries or testes occurs less frequently.9
In female patients, gonadal hormones, such as the luteinizing hormone (LH), estradiol, follicle-stimulating hormone (FSH), anti-mullerian hormone (AMH) and prolactin, are lower in transfusion-dependent thalassemia patients.7,10 Nearly half of these patients show a low to undetectable LH/FSH ratio.11 As a result, amenorrhea, anovulation and infertility are commonly found in adults. In younger females, the low gonadal function manifests as delayed puberty, delayed menarche or primary amenorrhea and short stature.12 In male patients, testicular functions are seen to be reduced, evidenced by the high anti-mullerian hormone and low testosterone levels.13 Lower sperm concentrations, a lower percentage of sperm with normal morphology, sperm DNA damage and reduced testis volume are also seen in transfusion-dependent thalassemia patients.14 The adult male clinical features are lack of facial and body hair, decreased muscle mass and appearance of fine facial wrinkles, gynecomastia, diminished libido, fatigue and ejaculatory dysfunction.15,16
Early assessment of gonadal dysfunction and its prompt treatment by chelation therapy may reduce its incidence, improve the quality of life in already burdened thalassemic patients and prevent further complications.17 Despite the high prevalence of thalassemia in our context, baseline information on gonadal function is not well addressed. Therefore, this study aimed to find the status of gonadal function in transfusion-dependent thalassemia patients and assess the role of the serum ferritin level in predicting hypogonadism.
MethodsStudy design, site & durationThis cross-sectional study was conducted from June 2020 to June 2021. Department of Transfusion Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, was selected as the study site, as it is located in the capital and receives patients from all over the country.
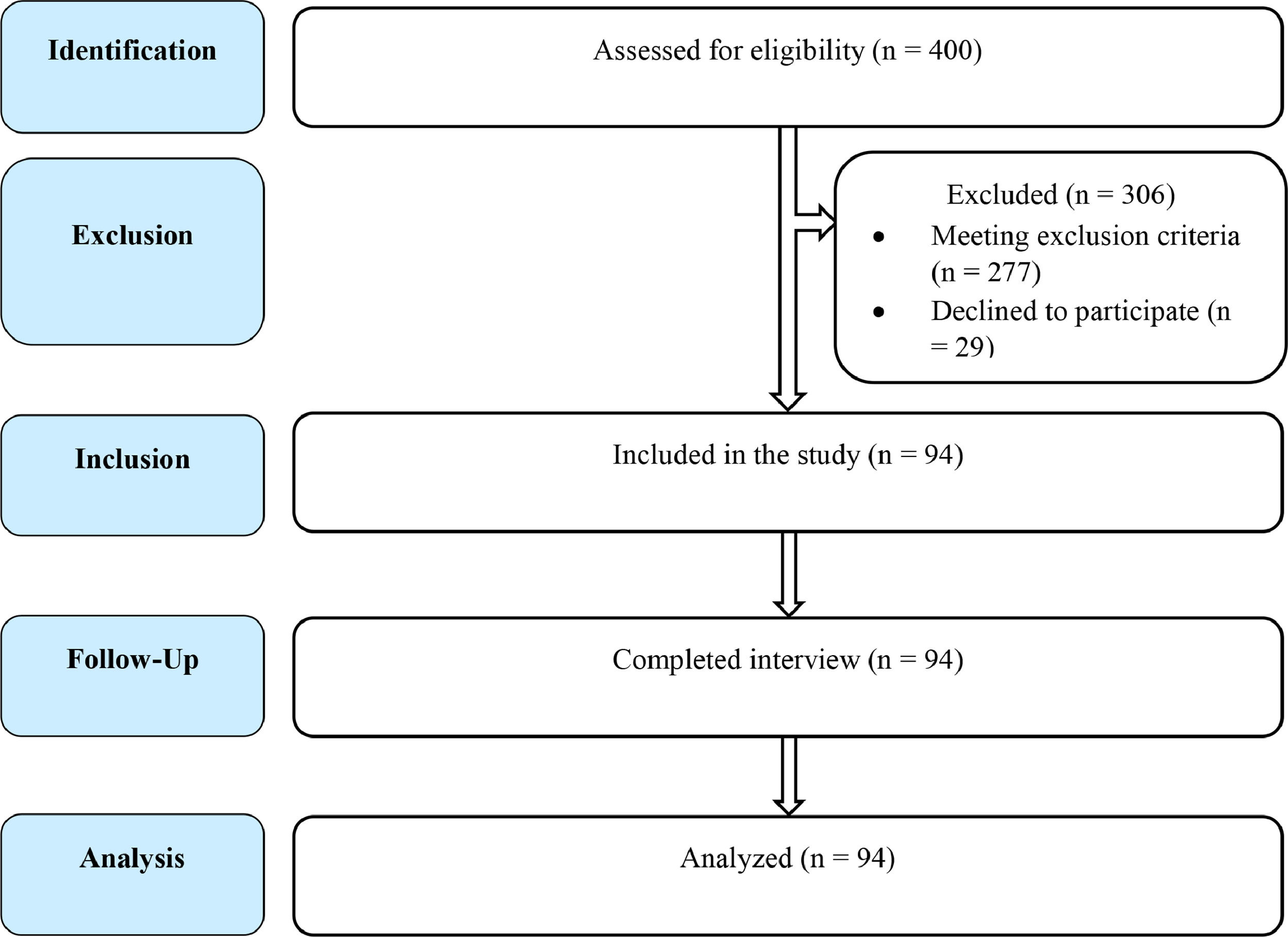
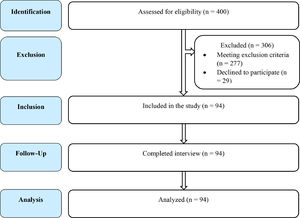
Study participantsβ-thalassemia patients diagnosed by hemoglobin electrophoresis, who were dependent on transfusion and providing consent, were included in the study. The β-thalassemia patients with other chronic illnesses that can cause gonadal dysfunction (e.g., connective tissue disease), under hormonal replacement therapy or taking an oral contraceptive pill (OCP), with congenital gonadal malformations, taking a regular antipsychotic, antidepressant and other drugs that hamper gonadal function, were excluded from the study. A total of 400 transfusion-dependent β-thalassemia patients, who visited the Department of Transfusion Medicine, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, during the study period, were approached for enrolment. Among these, 277 were excluded for meeting one or more of the exclusion criteria and 29 were excluded as they/their guardian did not provide consent to enter the study (Figure 1). Finally, a total of 94 patients having an average transfusion frequeny of 1 unit per month, of whom where 80 were β-thalassemia major (β°/β°)(85%) and 14, severe HbE/β-thalassemia (β°/HbE)(15%), were included in the study.
Data collectionThe data were collected by face-to-face interviews of patients/guardians using a pretested structured questionnaire. Background information, previous medical records, physical findings and laboratory reports were also assessed. Secondary sexual characteristics were assessed using the criteria proposed by Tanner.18 Patients in the pre-pubescent stage (stage 1 or stage 2) were classified as sexually undeveloped according to the Tanner staging. Patients in the pubescent (stage 3) or post-pubescent (stages 4 and 5) stages, on the other hand, were deemed sexually developed.19 The gonadal function of the patient was evaluated by history, physical examination and laboratory tests, including the serum luteinizing hormone (LH), serum follicle-stimulating hormone (FSH), serum testosterone for males and serum estradiol for females. The serum ferritin level was also measured to assess the iron overload status of the patients. Blood samples (3 - 5 ml) were collected from the patients, put into labelled test tubes and left for clotting, with all available aseptic precautions. The serum was then separated by centrifugation at 6,000 rpm after the blood had clotted. The assessment of hormone and ferritin levels was performed in the Department of Biochemistry and Microbiology, BSMMU, by the VEG-4000 Fully Automated ELISA Processor (manufactured by ESSE 3 Medical Equipment, Italy).
Statistical analysisAfter the data collection, the data were checked for errors and analyzed using the STATA (version 16). Continuous variables were presented as mean and standard deviation and categorical variables were presented as frequency and relative percentage. In addition, Pearson's chi-square test was performed to explore a bivariate relationship. A two tailed p-value of < 0.05 is considered statistically significant and all the reporting is performed according to the STROBE guidelines.20
EthicsThis study was approved by the Institutional Review Board of the BSMMU. The 1964 Declaration of Helsinki and later modifications and comparable ethical standards were followed, whenever feasible. Informed consent has been obtained from each participant/guardian.
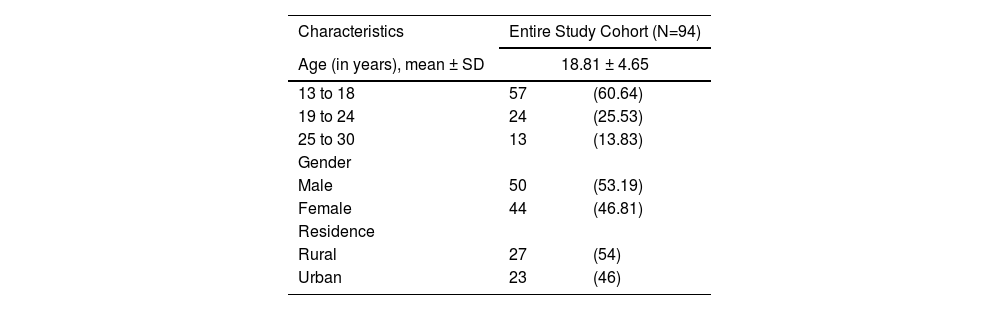
ResultsThe mean age of the participants was 18.81 ± 4.65 years, with the maximum being between 13 to 18 years of age (60.6%). There was a male (53.2%) preponderance, with a male-to-female ratio of 1.14:1. Most of the patients were from a rural area (57.4%) (Table 1).
Background information of study participants (n = 94).
Values are expressed as n(%), unless otherwise mentioned.
Abbreviations: SD, standard deviation.
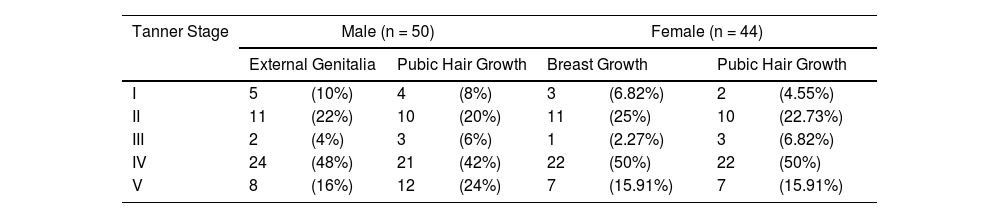
The most common features of sexual underdevelopoment among males were long downy pubic hair (22%) (Tanner stage II) and testes 2.5 to 3.2 cm (Tanner stage II). In the case of females, the prominent features of sexual underdevelopment were long downy pubic hair (10%) (Tanner stage II) and breast-budding (25%) (Tanner stage II) (Table 2).
Secondary sexual characteristics of study participants according to Tanner Staging (n = 94).
Values are expressed as n (%).
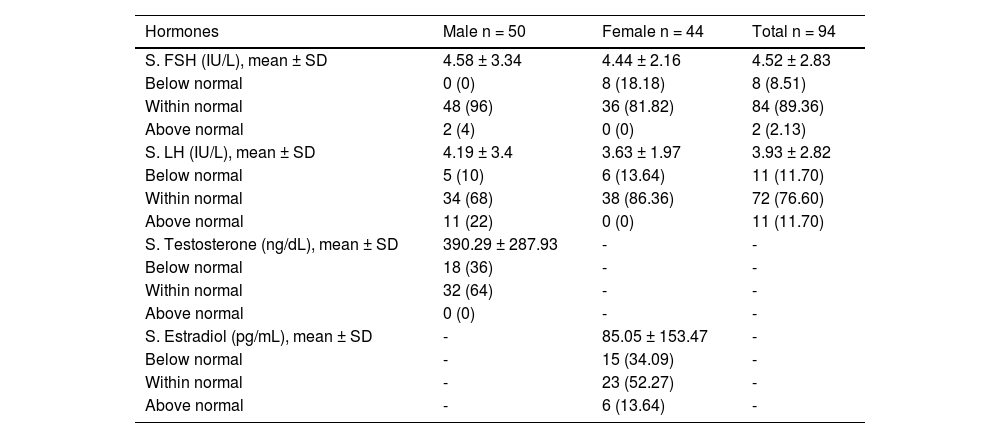
Among the 94 patients, the mean serum FSH of all patients was 4.52 ± 2.83 IU/L, of which the majority (89.36%) had a normal level of FSH and only 8.51% of the patients had an FSH below the normal level. Among the males, 4% had an FSH above the normal level, while none were below. Among females, 18.18% had an FSH below normal and none, above (Table 3). The mean serum LH of all the patients was 3.93 ± 2.82 IU/L, 11.70% having below the normal level of LH and the rest being within and above the normal. Among the males, 22% had an LH level above normal and 10%, below normal. Among the females, 13.64% had an LH below normal, while none were above normal. Among all the male patients, the mean serum testosterone was 390.29 ± 287.93 ng/dL, of which the majority (64%) had a normal testosterone level of and the rest (36%), below normal (Table 3). The mean serum estradiol of female patients was 85.05 ± 153.47 pg/mL, of which most (52.27%) had a normal level. Among the rest, 13.64% had above the normal level of estradiol and 34.09 %, below normal.
Laboratory findings for gonadal profile in study participants (n = 94).
Values are expressed as n (%), unless otherwise mentioned.
Abbreviations: S., serum; SD, standard deviation; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
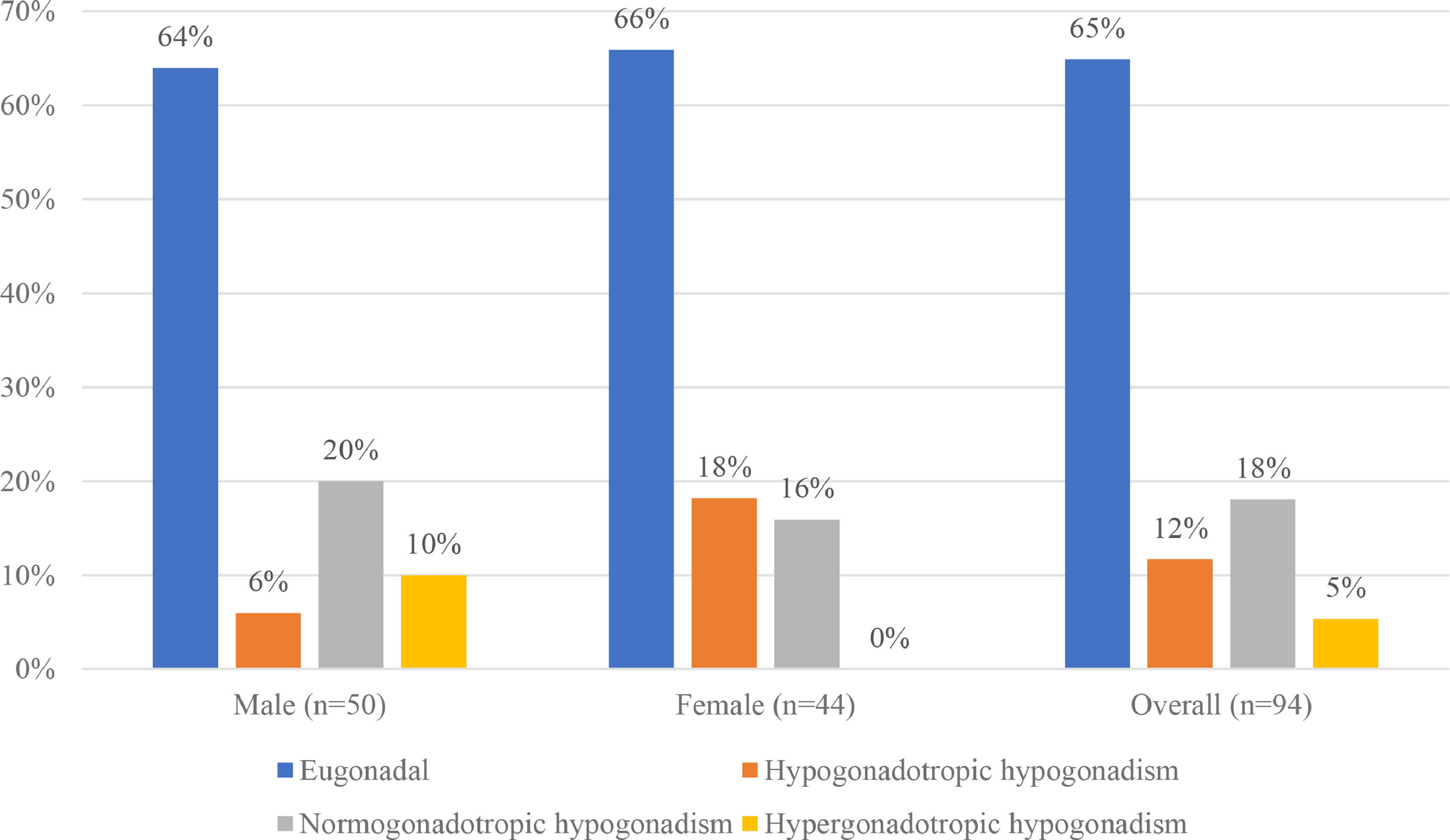
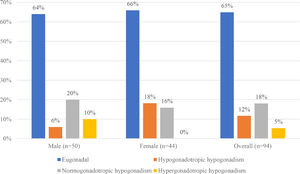
Hypogonadism was found in 33 patients (35.11%), afflicting 36% of all male and 34% of all female patients. Normogonadotropic hypogonadism, in 17 patients (18%), was the most prevalent type, followed by hypogonadotropic hypogonadism in 11 (12%) and hypergonadotropic hypogonadism in 5 (5%). The majority of the male patients with hypogonadism had the normogonadotropic type, found in 10 patients (20%); in contrast, the majority of the female patients with hypogonadism, 8 in all (18%), had the hypogonadotropic type. There was a significant difference in the distribution of the types of hypogonadism between males and females (p = 0.023) (Figure 2).
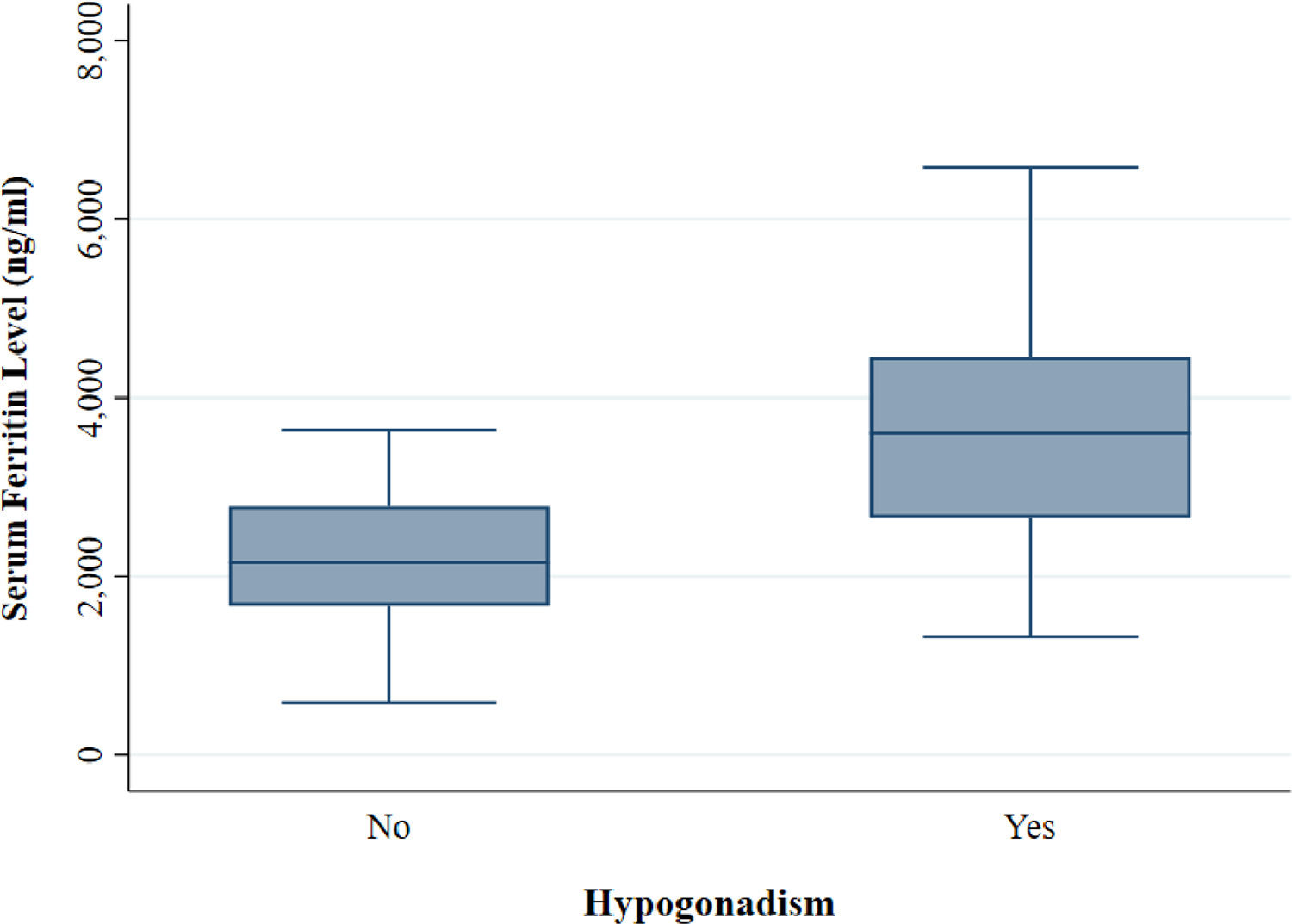
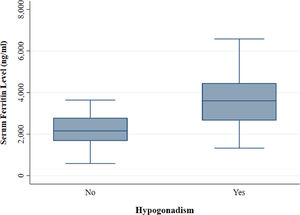
The mean serum ferritin level among the thalassemia patients was 2,665.51 (± 1,143.25) ng/ml. The serumferritin level was significantly higher (p < 0.001) in patients with hypogonadism (Eugonadal: 2,174.79 (±749.12) ng/ml; Hypogonadal: 3,572.59 (± 1,199.49) ng/ml) (Figure 3).
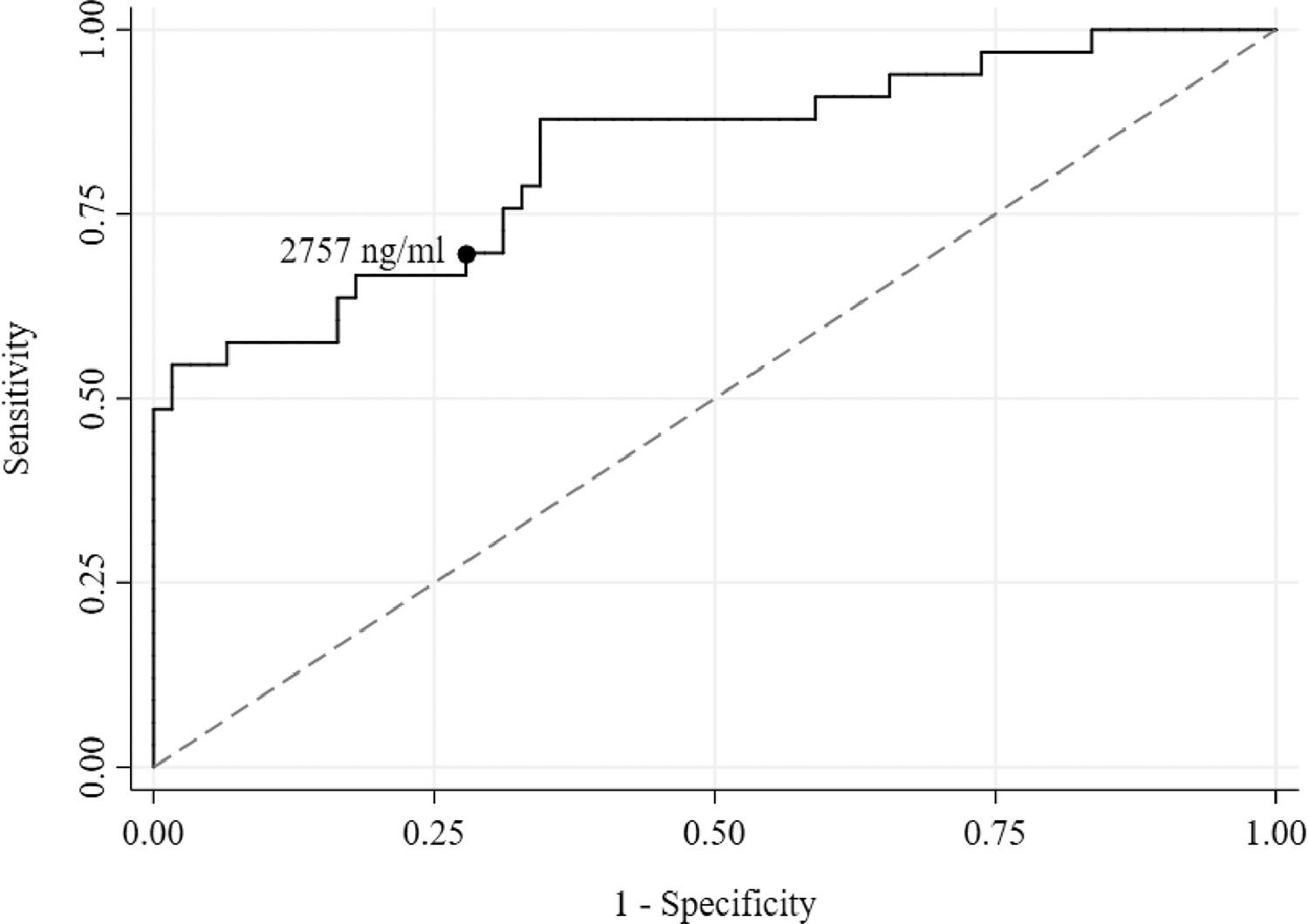
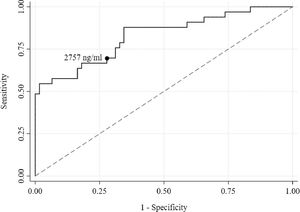
Using receiver operating characteristic (ROC) curve analysis, a serum ferritin of 2,757 ng/ml was found to be the best threshold (Sensitivity = 69.70% and Specificity = 72.13%) for discriminating the presence of hypogonadism (Figure 4). The area under the curve (AUC) was high (0.83) and the p-value was highly significant (< 0.001). Both lower and upper bound areas were also above the area of 0.5, indicating that serum ferritin level could accurately predict hypogonadism.
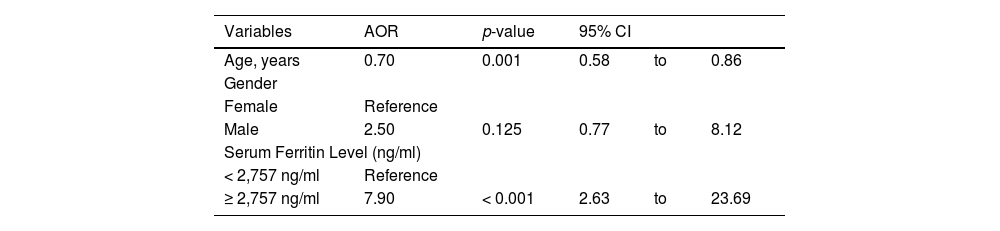
In the multiple logistic regression model, after adjusting for age and gender, patients with a serum ferritin level ≥ 2,757 ng/ml were eight times (AOR: 7.90, 95% CI: 2.63 to 23.69, p < 0.001) more at risk of developing hypogonadism, compared to those with a serum ferritin level < 2,757 ng/ml (Table 4).
Multiple logistic regression results for the factors associated with hypogonadism in thalassemia patients.
AOR, adjusted odds ratio; CI, confidence interval.
In our study, the overall prevalence of hypogonadism was 35.11%, 18.1% being normogonadotropic, 11.7% being hypogonadotropic and 5.3% being hypergonadotropic. In a study among 21 male patients with transfusion-dependent beta-thalassemia (TDT), the prevalence of hypogonadotropic hypogonadism was 33.3%, which is consistent with our study.21 Furthermore, a cohort study in Taiwan among 454 TDT major patients showed a lower prevalence of hypogonadism (23.1%).22 Another study carried out by Daraghmeh et al., in the thalassemia ward at the AL-Wattani hospital in Nablus among 75 TDT patients, found that hypogonadism was prevalent among 46.7% of the tested patients, including both primary and secondary hypogonadism.23 However, the prevalence of hypogonadism in this study population was lower than some published reports,7,24–27 all of which have reported hypogonadism as the most frequent endocrinopathy among TDT patients from different countries. The availability of iron chelation therapy can explain the lower prevalence of hypogonadism in our study, compared to other studies. A study on 382 TDT patients treated with desferrioxamine at the Thalassemia Centre in Dubai showed a significantly lower prevalence of hypogonadism of only 25%.28 Moreover, chelation therapy with desferrioxamine before puberty has helped patients attain normal sexual maturation in some studies. In a study of 40 patients with TDT, 90% of 19 patients who began treatment with desferrioxamine before the age of 10 years had normal sexual development, compared to only 38% of those treated after the age of 10 years.29 However, results of a prospective study in patients with thalassemia and secondary amenorrhea suggest that the damage to the hypothalamus is progressive, despite regular transfusion and chelation therapy.30 These results suggest that the development of hypogonadotropic hypogonadism might be caused by early and progressive damage due to iron loading. We believe that these could be attributed to the possible progressive toxic effect of iron-induced free radicals and/or to some other undefined risk factors involved in the development of hypogonadotropic hypogonadism.31
There was no statistically significant relationship between gender and hypogonadism in our study. This result was similar to the studies carried out in Iran and Palestine.32,33 However, this result was different from the results of a study by Dumaidi et al., which stated that a higher percentage of hypogonadism in males indicates that males are more prone to hypogonadism than females.27
In the current study, serum testosterone, estradiol, LH and FSH were lower among 36%, 34.09%, 11.70% and 8.51% of the patients, respectively. The most common symptom of gonadal dysfunction among males was the lack of axillary and pubic hair (6%) (Tanner stage II) and among females, slow or absent breast growth, hot flashes and amenorrhea (6.82% each). Regarding the menstrual status, adult females presented hypogonadism in the form of total absence of spontaneous menarche (primary amenorrhea); this applied to 29% of female participants with TDT, as confirmed by the low level of estradiol. In contrast, the other form is the lack or irregular menses (secondary amenorrhea), found in 42% of female participants with TDT. In males, hypogonadism was in the form of sexual infantilism; this applied to 72% of male participants with TDT, which agrees with contemporary studies.34,35 Several other studies also revealed that, in the thalassemic group, the baseline and peak levels, after the GnRH test, of luteinizing hormone (LH), follicle-stimulating hormone (FSH) and estradiol, were significantly lower than those in the control group.36,37 However, the discrepancy in the gonadotropin hormone level and gonadal dysfunction symptoms among males and females, compared to other studies, might be explained by ethnic factors, different availability of therapeutic agents and economic status.7
The transfusion-dependent beta-thalassemia (TDT) most often requires regular red blood cell transfusions, which start within the first year of life.38 The predilection for iron deposition in the pituitary gland and hypothalamus among these patients frequently causes a lack of sexual maturation and loss of gonadal function. Telfer et al. showed that the serum ferritin level is a relevant marker for the evaluation of iron overload.39 We found that hypogonadal patients had a significantly higher mean serum ferritin level, compared to eugonadal patients (Eugonadal: 2,174.79 (± 749.12) ng/ml; Hypogonadal: 3,572.59 (± 1,199.49) ng/ml). Patients with ≥ 2,757 ng/ml of serum ferritin level were almost eight times (AOR: 7.90, 95% CI: 2.63 to 23.69, p < 0.001) more at risk of developing hypogonadism, compared to those with a serum ferritin level < 2,757 ng/ml. This association between hypogonadism and serum ferritin level conforms to the existing evidence.40,41
ConclusionsIn our study, approximately one-third of the TDT patients were found to be suffering from different hypogonadisms. Hypogonadal patients had a significantly higher serum ferritin level and the serum ferritin level was highly accurate in predicting hypogonadism. Therefore, the serum ferritin level, along with gonadal hormone analysis, of TDT patients can be considered a screening tool for assessing gonadal function and early detection and prevention of hypogonadism. However, further prospective studies with a larger sample size might be necessary to provide better recommendations for patients with TDT.
We want to thank all the patients who participated in this study.