Collecting high-dose (HD) or double-dose (DD) apheresis platelets units from a single collection offers significant benefit by improving inventory logistics and minimizing the cost per unit produced. Platelet collection yield by apheresis is primarily influenced by donor factors, but the cell separator used also affects the collection yield.
ObjectivesTo predict the cutoff in donor factors resulting in HD and DD platelet collections between Trima/Spectra Optia and MCS+ apheresis equipment using Classification and Regression Trees (CART) analysis.
MethodsHigh platelet yield collections (target ≥ 4.5 × 1011 platelets) using MCS+, Trima Accel and Spectra Optia were included. Endpoints were ≥ 6 × 1011 platelets for DD and ≥ 4.5 to < 6 × 1011 for HD collections. The CART, a tree building technique, was used to predict the donor factors resulting in high-yield platelet collections in Trima/Spectra Optia and MCS+ equipment by R programming.
ResultsOut of 1,102 donations, the DDs represented 60% and the HDs, 31%. The Trima/Spectra Optia predicted higher success rates when the donor platelet count was set at ≥ 205 × 103/µl and ≥ 237 × 103/µl for HD and DD collections. The MCS+ predicted better success when the donor platelet count was ≥ 286 × 103/µl for HD and ≥ 384 × 103/µl for DD collections. Increased donor weight helped counter the effects of lower donor platelet counts only for HD collections in both the equipment.
ConclusionsThe donor platelet count and weight formed the strongest criteria for predicting high platelet yield donations. Success rates for collecting DD and HD products were higher in the Trima/Spectra Optia, as they require lower donor platelet count and body weight than the MCS+.
With increasing demand for platelet concentrates, collecting more than one apheresis platelet unit from a single donor offers benefit by strategically improving donor logistics and inventory implications. It also minimizes the cost of units produced by at least half and also helps in reducing operational costs.1 Platelet collection yield by apheresis is primarily influenced by donor-specific variables, but also by procedural differences and type of cell separator used.1 Multiple studies have demonstrated a positive correlation between pre-donation platelet count and product yield; however, not many studies are available to predict donor factors for achieving maximum yield from apheresis donors.2,3 Hence, it is important to target donors that are well-suited for maximizing platelet yield based on the apheresis equipment.
The US FDA requires a donor pre-donation platelet count of at least 150 × 103/µl and a post-donation platelet count target of no less than 100 × 103 platelets/µL to be set in the apheresis equipment during platelet collection.4 The AABB standards state that when the original apheresis unit is split into multiple units, each unit meet minimum standards of at least 3 × 1011 platelets in 90% of the sampled units.5 Units containing less than 3 × 1011 platelets should be labeled with actual yields.5 While the European guidelines specify 2 × 1011 platelets per unit as the acceptable adult dose,6 India has revised their standards in its latest Drugs and Cosmetics Act, establishing that single apheresis platelet concentrates should contain a minimum of 3 × 1011 platelets in 75% of the units tested among 1% of the monthly production, or 4 units per month, whichever is higher.7 Previous authors used their own definitions for labeling the apheresis platelet products as low-, standard- and high-dose.8,9
The Classification and Regression Trees (CART) analysis is a tree-building technique in which several predictor variables are tested to determine how they impact the outcome variable, such as overall survival and success rate.10,11 In India, very few studies discuss donors rendering a high platelet yield target per collection. The present study sought to identify the optimal cutoff in donor variables, such as donor age, weight, hematocrit and platelet count that predicts high-dose (HD) and double-dose (DD) platelet collections using the CART in Trima/Spectra Optia and MCS+ apheresis equipment in the Indian population.
MethodsStudy SettingThis was a retrospective study on platelet apheresis donors between 2016 and 2019 at a tertiary cancer center under the Government of Kerala, India. The blood center was the only center with an apheresis facility in the northern districts of Kerala catering to the needs of apheresis services. The blood center collects approximately 450 platelet donations annually and supports the bone marrow transplant program involving CD34 enumeration, stem cell harvest and cryopreservation. Approximately 20 transplants are performed at our center annually. Donors fulfilling the Indian criteria for whole blood donation were assessed for apheresis.12
Study populationDonors with a platelet count ≥ 150 × 103/µl and adequate venous access were counseled for high-dose and double-dose collections prior to apheresis, the operational definition for the high-dose (HD) platelet product being apheresis platelet products with a yield of ≥ 4.5 × 1011platelets and < 6 × 1011 platelets and for the double-dose (DD) product, being the apheresis platelet product with a yield of ≥ 6 × 1011platelets, leading to two adult dose units (each containing at least 3 × 1011platelets). Donors with a planned set target yield of < 4.5 × 1011 platelets were excluded from the analysis.
ProcedureThe blood center had the MCS+ (software version 2-UPP-A.2-IE, Haemonetics, Braintree MA, USA), Trima Accel, (software version 6.0, Terumo BCT, Lakewood, CO, USA) and Spectra Optia (Terumo BCT, Lakewood, CO, USA) equipment for platelet apheresis. The universal platelet protocol in the MCS+ equipment uses intermittent flow centrifugation in which the whole blood is pumped into the spinning bowl and platelet components are pushed upward and inward to the appropriate bag during each cycle. The system then concentrates the platelet product and suspends it in the platelet additive solution. The Trima Accel and Spectra Optia equipment works on continuous flow centrifugation and the leukoreduction system (LRS) chamber separates platelets from white blood cells using the elutriation principle. Donors underwent collection in one of these apheresis equipment. The donation endpoint, i.e., target yield, was set based on the estimated procedural time, donor comfort during the procedure, donor complications and estimated post-donation platelet count. The maximum procedure duration was set below 130 minutes for all the procedures. The blood center preferred donors with higher platelet counts for the MCS+ equipment due to the longer procedure duration.
Study parametersThe donor and procedural parameters, such as donor age, pre-donation platelet count, hematocrit, weight, procedure duration, software predicted platelet yield and actual platelet yield in product, were retrieved from apheresis records. As Trima Accel and Spectra Optia equipment use the principle of elutriation and continuous flow centrifugation, these procedures were combined under a single category Trima/Spectra Optia and analyzed.
The apheresis products were tested for quality control with a minimum of 4 units per month, or 1% of the monthly collection. The complete blood count of donors and apheresis platelet products were performed on the LH 750 (Beckman Coulter, California, USA) or on the Medonic M32M (Boule Diagnostics AB, Sweden) hematology analyzer. The laboratory performs three level quality control steps for the hematology analyzer daily. To test the software predicted platelet yield against actual platelet yield, the linear regression model was used to build the mathematical model for the actual platelet yield.
The CART analysis was performed using the R programming software, in which the CART analysis recursively partitions observations in a matched data set, consisting of a categorical (for classification trees) or continuous (for regression trees) dependent variable (i.e., software predicted platelet yield for HD and DD platelet donations) and one or more explanatory donor factors (weight, age, hematocrit and platelet count), into progressively smaller groups. Each partition is a binary split. During each recursion, splits for each donor factor are examined and the split that maximizes the homogeneity of the two resulting groups, with respect to the HD and DD platelet donation in Trima/Spectra Optia and MCS+ apheresis equipment, is chosen.
Statistical MethodsData was entered in the MS excel sheet and validated by the investigators. All discrepancies in the data were verified by confirming with apheresis records. The statistical analysis was performed using the Statistical Software IBM SPSS Statistics for Windows, Version 20.0 Armonk, NY: IBM Corp. and R programming. The descriptive statistics, such as the mean and SD were calculated to describe the study variables. The Mann Whitney U test was used to study the association between the HD/DD platelet yield and donor characteristics. A p-value of < 0.05 was considered significant.
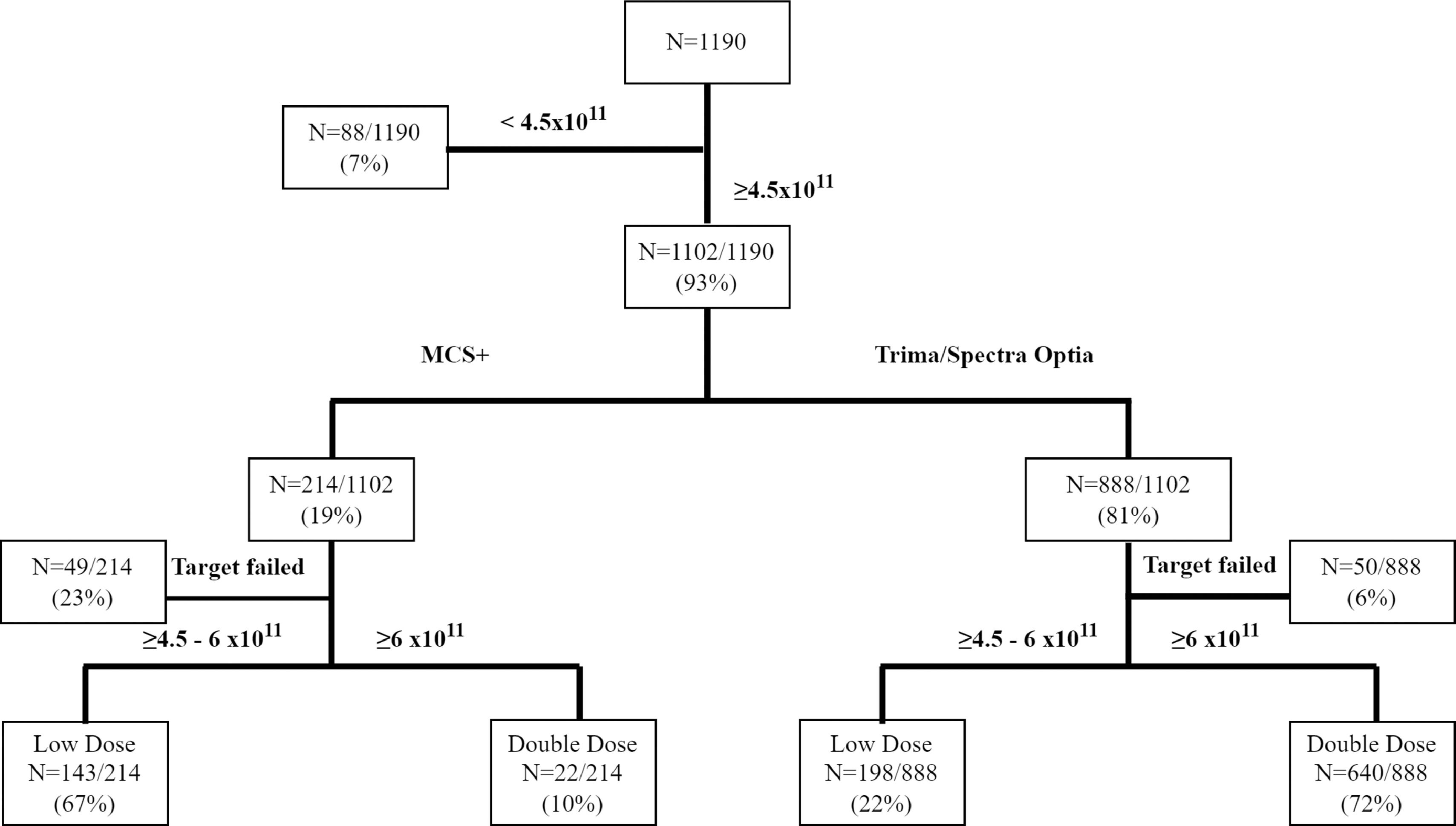
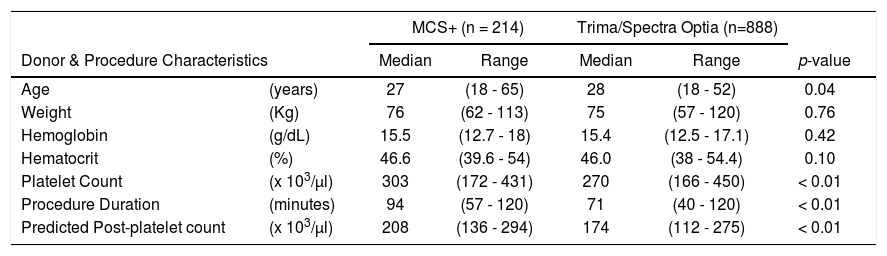
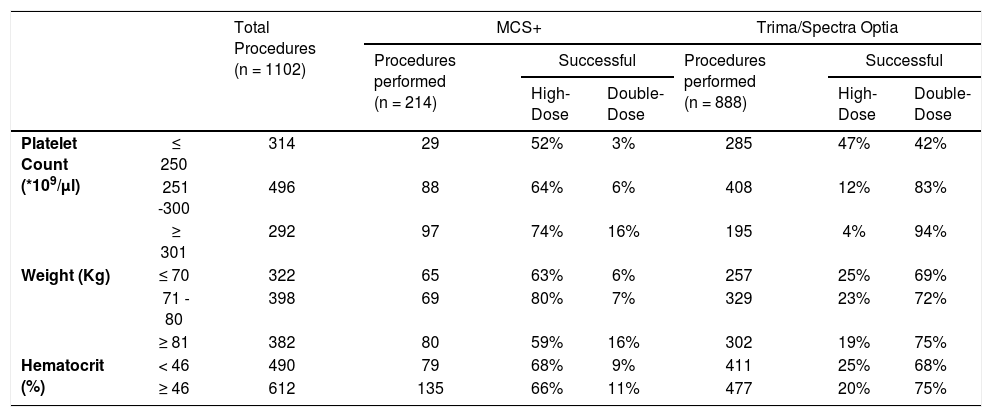
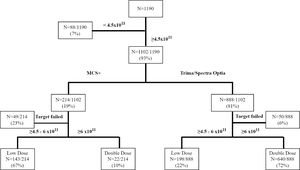
ResultsA total of 1,190 platelet apheresis procedures were performed during the study period. For the CART analysis, the data was derived from 1,102 platelet collections, excluding the 88 procedures planned with a platelet yield < 4.5 × 1011 (Figure 1). All donors who donated HD and DD platelets were males. Table 1 shows the distribution of donor and procedural characteristics between MCS+ and Trima/Spectra Optia equipment. Overall, donors analyzed by the MCS+ equipment had a higher baseline platelet count, predicted post donation platelet count and procedure duration than those by the Trima/Spectra Optia equipment (Table 1).
Comparison of baseline donor characteristics and procedure details in high platelet yield apheresis between MCS+ and Trima/Spectra Optia devices.
The DD platelet products were obtainable in 662 (60%) collections and the HD products, in 341 (31%). A total of 99 (9%) procedures resulting in platelet yield < 4.5 × 1011 during the collection were aborted, as shown in Figure 1. Table 2 shows the distribution of donor parameters resulting in successful HD and DD products between the equipment. In donors with a platelet count < 250 × 103/µl, the distribution of successful HD collection was similar between both devices (MCS+: 52%; Trima/Spectra Optia: 47%). However, within donors with platelet counts < 250 × 103/µl, successful DD collections were possible in 42% in the Trima/Spectra Optia, while the MCS+ had only 3%. In the MCS+, the percentage of successful procedures for HD collections improved with an increase in the donor platelet count, while for the same donor count, the Trima/Spectra Optia was able to successfully collect higher DD collections (Table 2). Overall, the successful rate in HD and DD did not differ, as per donor body weight and hematocrit, between the devices.
Distribution of % successful high-dose and double-dose platelets collected among donor characteristics between MCS+ and Trima/Spectra Optia devices.
The CART analysis was applied to build two types of algorithms to predict the success rates (Rs) for collecting HD or DD products between the MCS+ and Trima/Spectra Optia apheresis equipment.
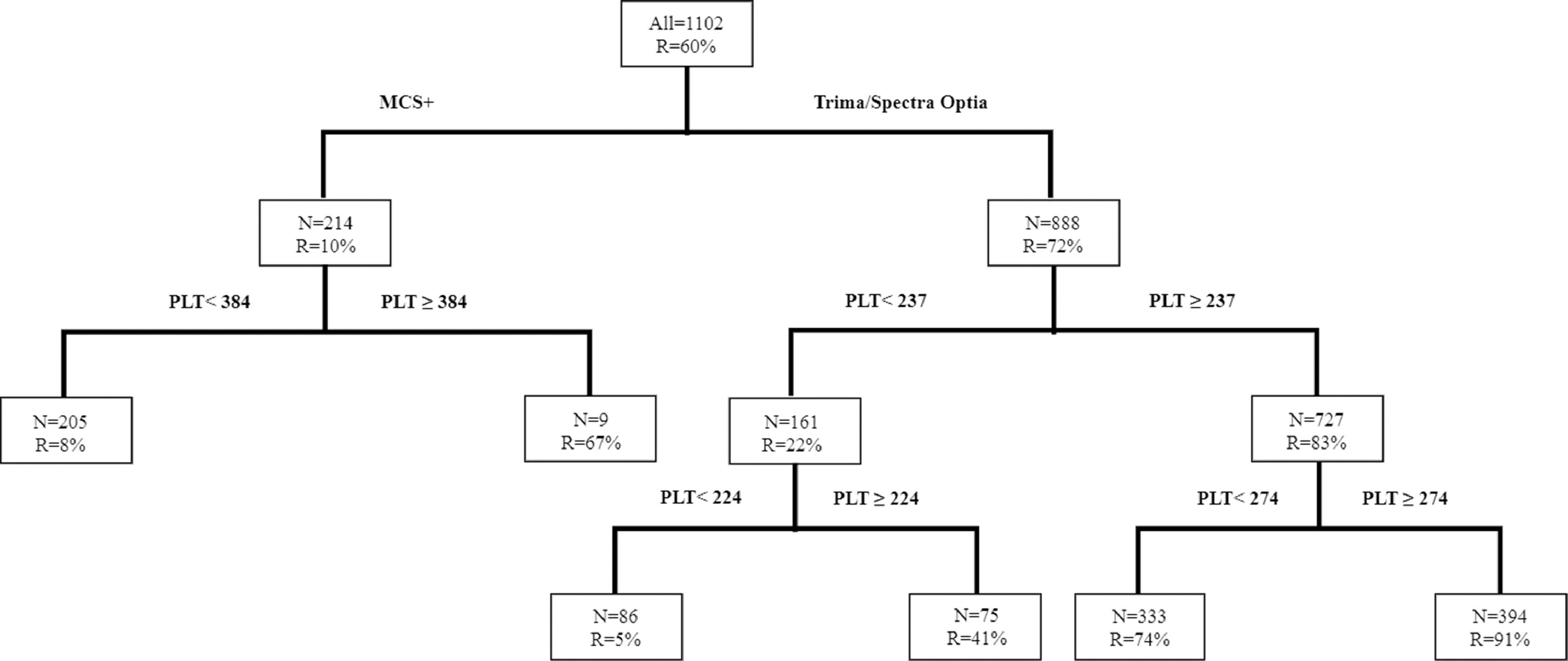
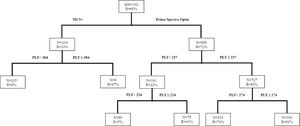
CART for high-dose & double-dose platelet yieldFigure 2 shows the CART analysis for DD platelet collection; the overall success rate (R) was 60%. The analysis identified the donor platelet count as the main predictor for collecting DD platelets. In the Trima/Spectra Optia, donors with a platelet count ≥ 237 × 103/µl had an R of 83% for DD collections and an R reduced to 22% with a platelet count < 237 × 103/µl. Similarly, among the donors with a platelet count ≥ 237 × 103/µl, the R improved to 91%, when donors had a platelet count ≥ 274 × 103/µl. The chance of a DD platelet collection with the Trima/Spectra Optia dropped to R = 5% when donors had a platelet count < 224 × 103/µl. On the other hand, for DD platelet collections, the MCS+ equipment only had an overall R = 10%, which improved to R = 67% when donors had a platelet count ≥ 384 × 103/µl.
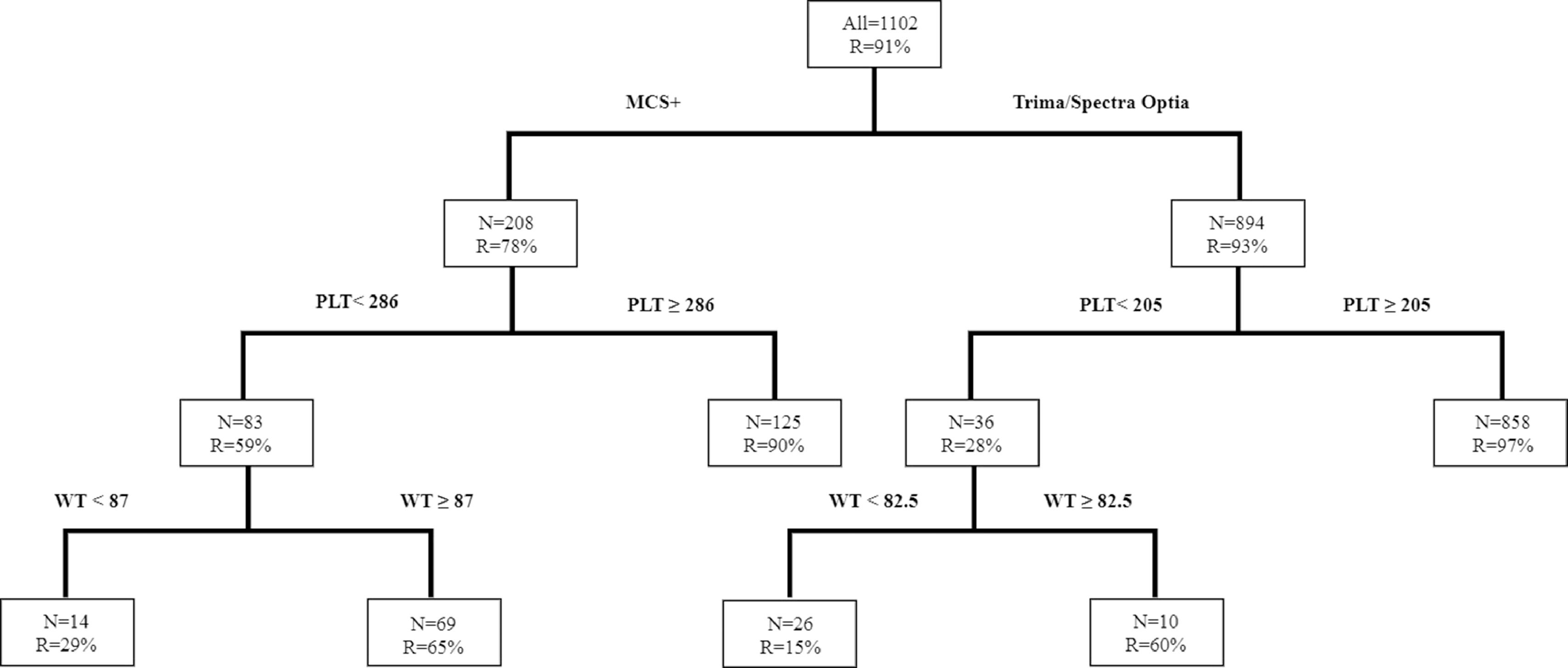
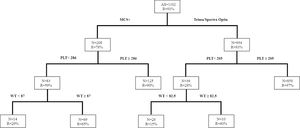
Similarly, for HD platelet collections, the study identified that the Trima/Spectra Optia had a higher overall success rate, compared to the MCS+ (R = 93% vs. 76%). Figure 3 shows that when the donor platelet count was ≥ 205 × 103/µl and donating with the Trima/Spectra Optia, the success rate (R) was 97%, while for donors with a platelet count < 205 × 103/µl, the success rate improved if the donor weight was ≥ 82.5kg (R = 60% vs. 15%) with the Trima/Spectra Optia. Donors donating with the MCS+ had a better success rate when the platelet count was ≥ 286 × 103/µl (R = 86% vs. 59%). Similarly, in the MCS+ with a donor platelet count < 286 × 103/µl, the body weight at 87kg was the best discriminator, as the success rate improved in donors weighing ≥ 87kg (R = 65% vs. 29%).
Software versus actual product platelet yieldThe actual product yield was available for 230 procedures and showed a very high positive correlation (r = 0.77; p < 0.01) with the software-predicted yield. The linear regression model was performed to predict the actual yield from the software-predicted yield (R2 = 0.591). The simplified equation for determining the actual yield would be -0.202+ (0.998*software-predicted yield).
DiscussionBlood centers which deal with high platelet collection yields either transfuse all of the platelets to one patient, or split the product to treat two or more patients.13 At present, there are no established pre-defined cutoffs in donors that determine a high platelet yield, such as HD or DD collections. The study showed that the donor platelet count is the best predictor for collecting high platelet yield products, coinciding with previous studies. The Trima/Spectra Optia had a higher success rate than the MCS+ in both HD and DD platelet yield collection.
High platelet yield collection has improved transfusion practices by minimizing the patient exposure to infections and alloimmunization. When collected as DD, it also has improved the logistics of donors required per patient and reduced the cost per transfusion.1 With new technologies in apheresis, blood centers aim to achieve the maximum platelet yield in the minimal procedure time. A previous study had observed that both the MCS+ and Trima performed plateletpheresis quickly and efficiently.14 Hence, optimizing platelet collection with the available apheresis equipment and donor factors, such as blood counts and biometrics, is essential.
The donor platelet count was used as a predictor for DD platelet donations in earlier studies. In their retrospective study, Vassallo et al. observed a linear relationship between the donor pre-platelet count and platelet collection yield for a fixed volume of blood processed.13 In the present study, utilizing CART in the Trima/Spectra Optia equipment, a higher success rate was obtained when the donor platelet count was set at ≥ 205 × 103/µl and ≥ 237 × 103/µl for HD and DD product collections, respectively. Donor body weight ≥ 82.5kg improved the success rate when the donor platelet count was < 205 × 103/µl for HD when collected with the Trima/Spectra Optia. The MCS+ equipment also predicted better success when the donor platelet count was ≥ 286 × 103/µl for HD and ≥ 384 × 103/µl for DD collections. The increased donor weight helped counter the effects of lower donor platelet counts in the high platelet yield collections and vice versa.3 Additionally, the donor body weight at ≥ 87kg had improved their success rates for HD collections when the donor platelet count was < 286 × 103/µl in the MCS+. Wollershiem et al. focused on donor characteristics such as pre-count, hematocrit and body weight with higher platelet yields in the Trima equipment prospectively. They observed that when the criterion for donor platelets was set at > 225, 82% of the procedures yielded DD products, compared to 54% of the procedures with a pre-count < 225 (p < 0.01). Moreover, donor weight > 65 kg also resulted in good outcomes for DD products in their population.2
Woodall et al were the first to utilize the CART algorithm in donor apheresis retrospectively and observed donors factors, such as donor weight ≥ 75.7 kg, yield twice the chances of donating DD platelets than that < 75.7 kg.1 It was observed that among donors weighing < 75.7 kg, the female sex was twice as likely to donate DD platelets as males.1 Donors with a platelet count ≥ 216 appear twice as likely to donate DD platelets than those with a platelet count < 216 in the Amicus equipment. However, in the present study, the relationship between sex and high-yield platelet donation was not determined, as only male donors underwent high platelet yield apheresis. In their retrospective study, Jamie Perez et al. predicted donor platelet count ≥ 230 as the optimal cutoff for DD platelet donations using the Trima equipment by regression analysis.3
In the present study, the distribution of donor factors did not influence the HD or DD platelet collections in the univariate analysis. In the CART analysis, the donor body weight did not seem to affect the success rate for DD collections, but influenced HD collections in both devices. Chellaiya et al. retrospectively observed that donors with a low hematocrit required higher blood volume processing with the MCS+ equipment and not with the Trima/Spectra Optia.15 However, in the present study, neither the hematocrit nor donor age provided a better split success rate with the CART algorithm between these devices.
The apheresis-induced depletion of the donor platelet count represents one of the main safety constraints for collecting high platelet yield products.16 Earlier studies had shown that almost one-third of platelets are in the exchangeable pool in the spleen and the rest remains in circulation in a normal person.1 Fontana et al. further observed that one-third of the platelets collected during apheresis were recruited from the spleen.16 Recent apheresis equipment, such as the Trima/Spectra Optia, measures the splenic release of platelets, while estimating the post-procedure platelet count in donors. Hence, for high-yield platelet collection, the accuracy of the apheresis equipment, that it does not deviate from the targeted yield, is extremely important. Jamie Perez et al. observed that the platelet yield calculated by the Trima/Spectra Optia v6.0 cell separator software consistently underestimated the actual platelet yield in our donors. They explained that the software prediction cutoff of 4.65 × 1011 was sufficient to obtain a DD platelet donation.3
The present study observed a high positive correlation between the actual product platelet yield and the software-predicted yield (r = 0.77) and differences between the both have been reported by other studies as well.3 This forms one of the potential limitations to our study, as we used the software-predicted yield for developing the CART algorithms. Due to its retrospective nature, the selection bias was very prominent in the present study, with donors with higher platelet counts being selected for the MCS+ equipment. However, this preliminary study will help identify donors suitable for HD and DD collections at our institution, based on their characteristics. Future prospective or randomized trials can reduce selection bias and help validate the CART algorithm. The other limitations, being donor complications resulting in the termination of the procedure before reaching the target yield, were not discussed.
In India, only a few studies have discussed double-dose platelet collections. Makroo et al. and Chaudary et al. had studied the donor characteristics and complication rates of double-dose platelet collections by setting the target yield at 6 × 1011 and 5.5 × 1011, respectively, during plateletpheresis.17,18 With the shrinking apheresis donor pool, donor comfort during high platelet yield donations remains an important tool for donor retention and repeat donations. The donor platelet count and body weight represent the strongest criteria for predicting high platelet yield donations. Our study results and those of previously reported studies emphasize that the differences in donor characteristics and equipment features impact the high platelet yield collections.16
ConclusionThe donor platelet count appeared as the strongest predictor for collecting HD and DD platelet products in both the Trima/Spectra Optia and MCS+ devices, while the donor weight predicted limited success rates only in HD collections. The donor age and hematocrit did not affect the success rate in either of the devices in the CART algorithm. The success rate for HD collections were better when the donor platelet count was ≥ 205 × 103/µl for the Trima/Spectra Optia and ≥ 286 × 103/µl for the MCS+ equipment. Similarly, for DD platelet collections, the success rate dropped when the donor platelet count was < 237 × 103/µl for the Trima/Spectra Optia and < 384 × 103/µl for the MCS+ equipment. Hence, it is advisable to choose the apheresis equipment which matches the donor factors when performing HD or DD platelet collections.
DeclarationsFunding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Availability of data and material: Not applicable.
Code availability: Not applicable.
Ethical Approval: Being a retrospective study, the Institutional Review Board (IRB) approved the present study without ethical approval through Ref. No: 1616/IRB-SRC/13/MCC/26-10-2019/1.
Consent to participate: Not applicable.
Consent for publication: Not applicable.
The authors acknowledge Soumya Das and Merline Augustine for proofreading the manuscript.