High-dose chemotherapy with autologous hematopoietic stem cell transplantation (auto-HSCT) improved 5-year overall survival rates in relapsed/refractory germ cell tumors (GCTs) from 10% to 52%. Nearly 30% of GCT patients are deemed poor mobilizers after receiving several lines of prior therapy. There is limited data available regarding upfront plerixafor use in GCT patients. We predicted upfront plerixafor use would increase the amount of stem cells collected preventing subsequent mobilizations and improve time to curative therapy. A retrospective, single center, chart review of adult GCT patients who received plerixafor upfront for mobilization at a single center between January 1, 2013 and August 31, 2021 was performed.
The primary objective was to evaluate the rate of successful peripheral blood CD34+ cell collections. Secondary objectives consisted of describing the impact of plerixafor use on mobilization and assessing auto-HSCT related outcomes. Sixteen patients received plerixafor upfront after an average of three prior lines of therapy (range: 2-5 lines). Successful collection (≥4 × 106 CD34+ cells/Kg collected within four days) was achieved in 15 (94%) patients in a median of one apheresis day (interquartile range: 1-2 days). All patients proceeded to an initial auto-HSCT and 12 patients (75%) completed both transplants as planned. Survival at 12 months was 50%.
The significantly higher amount of CD34+ cells collected over less apheresis days demonstrated the clinical utility of upfront plerixafor and its potential to facilitate more efficient stem cell mobilization. There is a need for larger randomized studies with upfront plerixafor use in this unique patient population.
Germ cell tumors (GCTs) are among the most curable solid malignancies with a five-year survival rate of over 95 percent.1 However, a small proportion (5-10%) are diagnosed with primary metastatic disease and approximately 30% of these patients will experience relapse or refractory disease and require further therapy.2 Even with salvage conventional-dose chemotherapy response rates are only 15-70% with long-term outcomes having a median progression-free survival of 9.8 months and median overall survival of 41 months.2 This highlights the importance of single or sequential high-dose chemotherapy with autologous hematopoietic stem cell transplant (auto-HSCT) in non-responders to upfront chemotherapy which has shown significantly superior progression-free survival at 2 years (hazard ratio - HR: 0.44; 95% Confidence interval – 95% CI: 0.39-0.51) and improved 5-year overall survival (HR: 0.65; 95% CI: 0.56-0.75) compared to conventional-dose chemotherapy.3,4 In a retrospective analysis of 1984 patients with relapsed GCT, the use of single or sequential high-dose chemotherapy and auto-HSCT demonstrated an overall 56% decrease in the risk of progression after first salvage treatment compared to those who received conventional-dose chemotherapy.4 Those with relapsing or refractory GCTs have often failed several lines of treatment that can impede stem cell reserve and make stem cell collection a challenge.5 Other risk factors are previous extensive radiation therapy of the bone marrow and treatment with alkylating agents, therefore, nearly 30% of GCT patients are deemed poor mobilizers (defined as failure to generate at least 2 million CD34+ cells/Kg per transplant).5-8 Furthermore, most high-dose chemotherapy protocols utilized in patients with GCTs often require peripheral blood stem cell rescue with at least approximately 4 million CD34+ cells/Kg to support sequential transplants.7 The two most common mobilization strategies in the literature for this population are granulocyte colony-stimulating factor (G-CSF) alone or G-CSF combined with chemotherapy.7 Due to the risk of hematopoietic cell mobilization failure in this patient population, additional methods for enhancing mobilization are needed.
Another strategy commonly used for hematopoietic cell mobilization is plerixafor plus G-CSF. However, it is only approved for the mobilization of hematopoietic stem cells for collection and subsequent auto-HSCT in patients with non-Hodgkin's lymphoma and multiple myeloma.9 This was based on two phase III, multicenter, randomized, double-blind, placebo-controlled trials evaluating the safety and efficacy of plerixafor plus G-CSF for mobilization in multiple myeloma and non-Hodgkin's lymphoma that found a significantly greater proportion of patients achieved successful mobilization compared to a placebo plus G-CSF (71.6% vs. 34.4%; p-value <0.001 and 59.3% vs. 19.6%; p-value <0.001, respectively).10-12 When compared to chemomobilization plus G-CSF in these same populations, plerixafor use also had significantly lower failure rates highlighting its superior efficacy for mobilization.8,9 Mechanistically, plerixafor is a slowly reversible inhibitor of CX chemokine receptor 4 (CXCR4) and it interacts with stromal derived factor-1α (SDF-1α) which regulates survival, trafficking, and homing of hematopoietic stem cells in the bone marrow.13 This inhibition leads to mobilization of CD34+ cells into the peripheral blood, even in cases where prior mobilization attempts have failed.13 Timing wise, plerixafor is recommended to be initiated at least four days after the initiation of G-CSF administration and approximately 11 hours prior to each apheresis session at a dose of 0.24 mg/Kg once daily for up to four consecutive doses.9
Several small studies have evaluated the use of plerixafor for GCTs as a salvage mobilization strategy for patients who previously failed mobilization as well as a preemptive approach in patients deemed high risk for mobilization failure.8,14-17 Among the retrospective analyses, successful mobilization rates ranged from 66-100% with the use of plerixafor plus G-CSF for remobilization in patients with GCTs who previously failed collection.8,15,16 Despite promising efficacy, plerixafor use in GCTs is not widely studied or approved for use in this population. At our institution, plerixafor is routinely offered upfront for GCT patients undergoing stem cell mobilization in an effort to achieve greater yield of stem cells in fewer days of apheresis. The aim of this study was to evaluate outcomes associated with upfront plerixafor administration for mobilization in all patients with GCTs undergoing high-dose chemotherapy and auto-HSCT.
Materials and methodsA retrospective analysis of data related to stem-cell mobilization was conducted at a university-based cancer center of adult GCT patients who underwent high-dose chemotherapy with auto-HSCT from January 1, 2013 to August 31, 2021. This project was approved by the University of Miami System Institutional Review Board. Patients were identified through the Center for International Blood and Marrow Transplant Research (CIBMTR) database and data on demographics, baseline characteristics, pertinent medical history, stem cell mobilization, apheresis and auto-HSCT were collected from their electronic medical records.
The primary objective was to evaluate the rate of successful peripheral blood CD34+ cell collection, defined as ≥ 4 × 106 CD34+ cells/Kg collected within four days of apheresis. Secondary objectives consisted of describing the impact of plerixafor use on mobilization and of assessing auto-HSCT related outcomes. Baseline characteristics and all outcomes are summarized descriptively.
All patients undergoing mobilization received G-CSF at a dose of 10 mcg/Kg subcutaneous on Days 1 through 4 with plerixafor added at a dose of 0.24 mg/Kg subcutaneous in the evening of Day 4 prior to the initiation of apheresis the following morning. Additional doses of G-CSF and plerixafor were utilized for patients requiring more than one apheresis session to achieve successful stem cell collection. Large volume leukapheresis was performed following institutional guidelines.
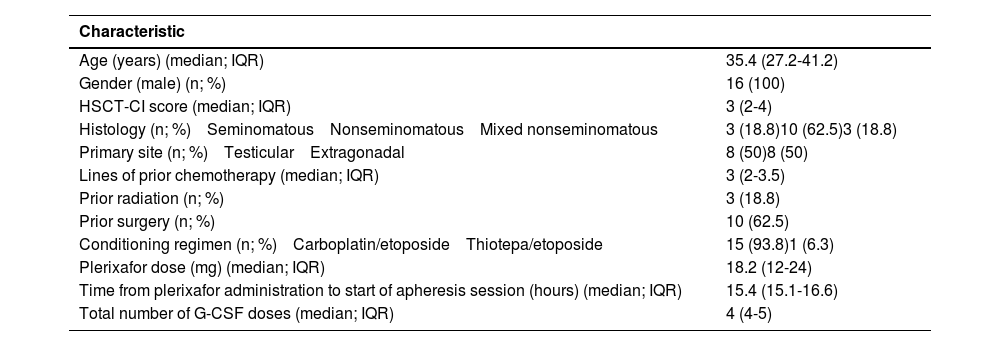
ResultsSixteen patients who received upfront plerixafor were included; ten had nonseminomatous, three had mixed nonseminomatous and three had seminomatous GCTs. The median age was 35 years old (interquartile range - IQR: 19-53 years). Four patients that did not receive plerixafor during mobilization were excluded. Baseline characteristics of the patients are summarized in Table 1. The majority of patients received a median of three lines of prior chemotherapy (IQR: 2-3.5 lines of prior chemotherapy).
Baseline characteristics of the 16 patients.
IQR: Interquartile range; G-CSF: granulocyte colony-stimulating factor
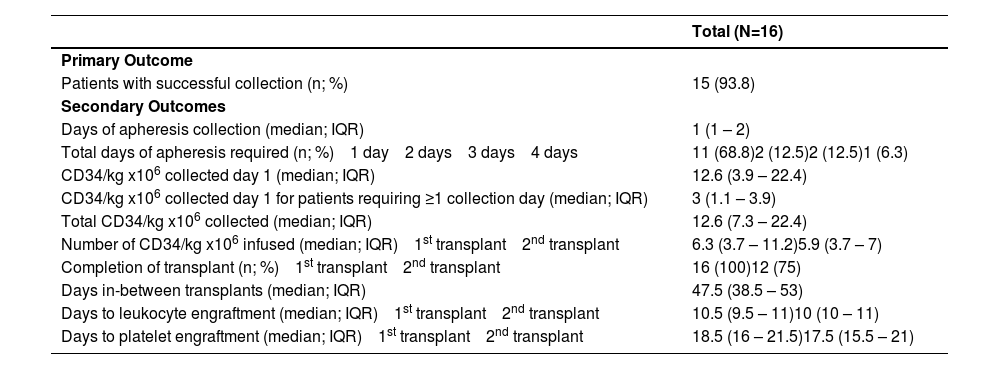
Successful collection was achieved in 15 (94%) patients with a median of one apheresis per day (IQR: 1-2 days). One patient was unable to successfully collect based on the definition of ≥ 4.0 × 106 CD34+ cells/Kg after four days of apheresis, but still proceeded to transplant with 3.57 × 106 CD34+ cells/Kg collected. A median total of 12.6 × 106 CD34+ cells/Kg (IQR: 7.31-22.37 × 106 CD34+ cells/kg) were collected. Among the five patients who required more than one apheresis session, a median total of 4.66 × 106 CD34+ cells/Kg were collected compared to a median total of 14.15 × 106 CD34+ cells/Kg among patients requiring only one apheresis session. A median of 29.5 liters were processed per apheresis session (IQR: 25.2-32 liters). All patients proceeded to auto-HSCT and 12 patients (75%) completed tandem transplants as planned. Across both transplants, the median time to neutrophil engraftment was ten days (IQR: 10-11 days) and to platelet engraftment it was 18 days (IQR: 16-21 days), (Table 2).
Primary and secondary outcomes.
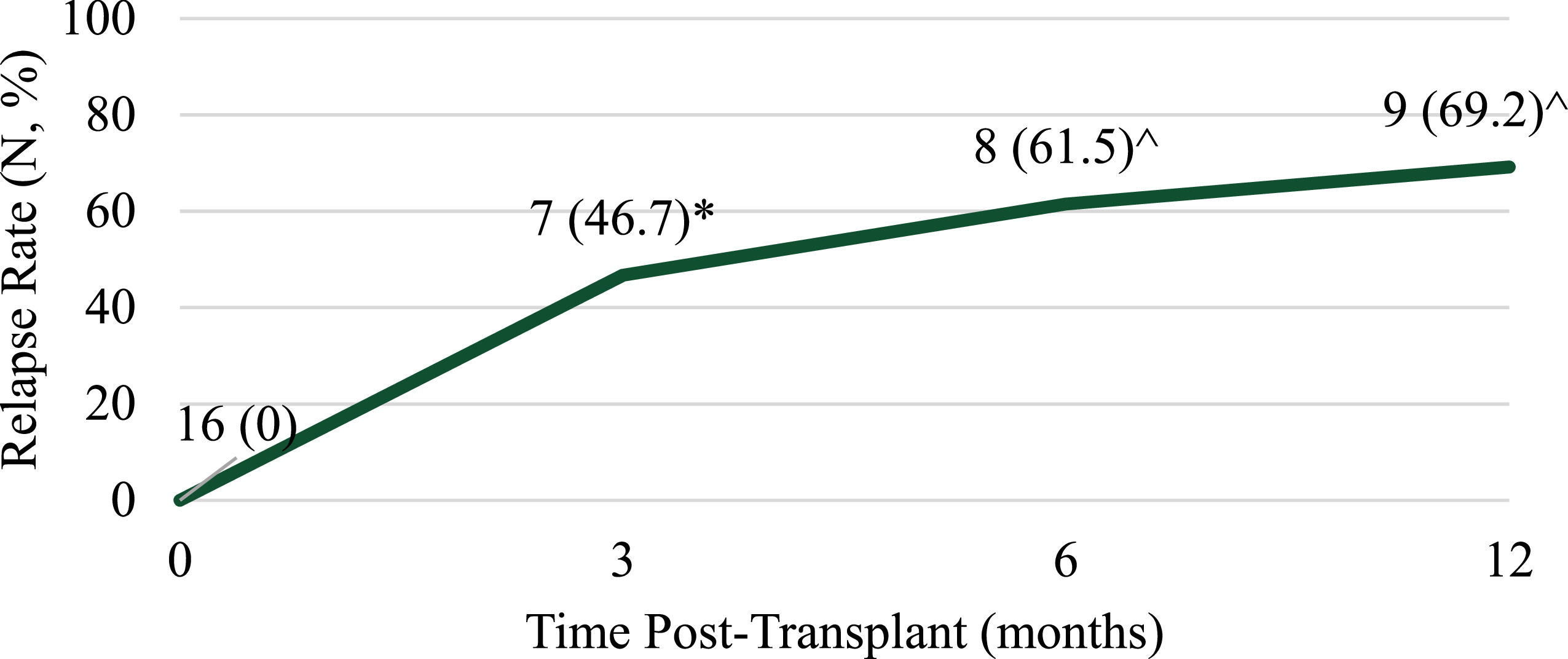
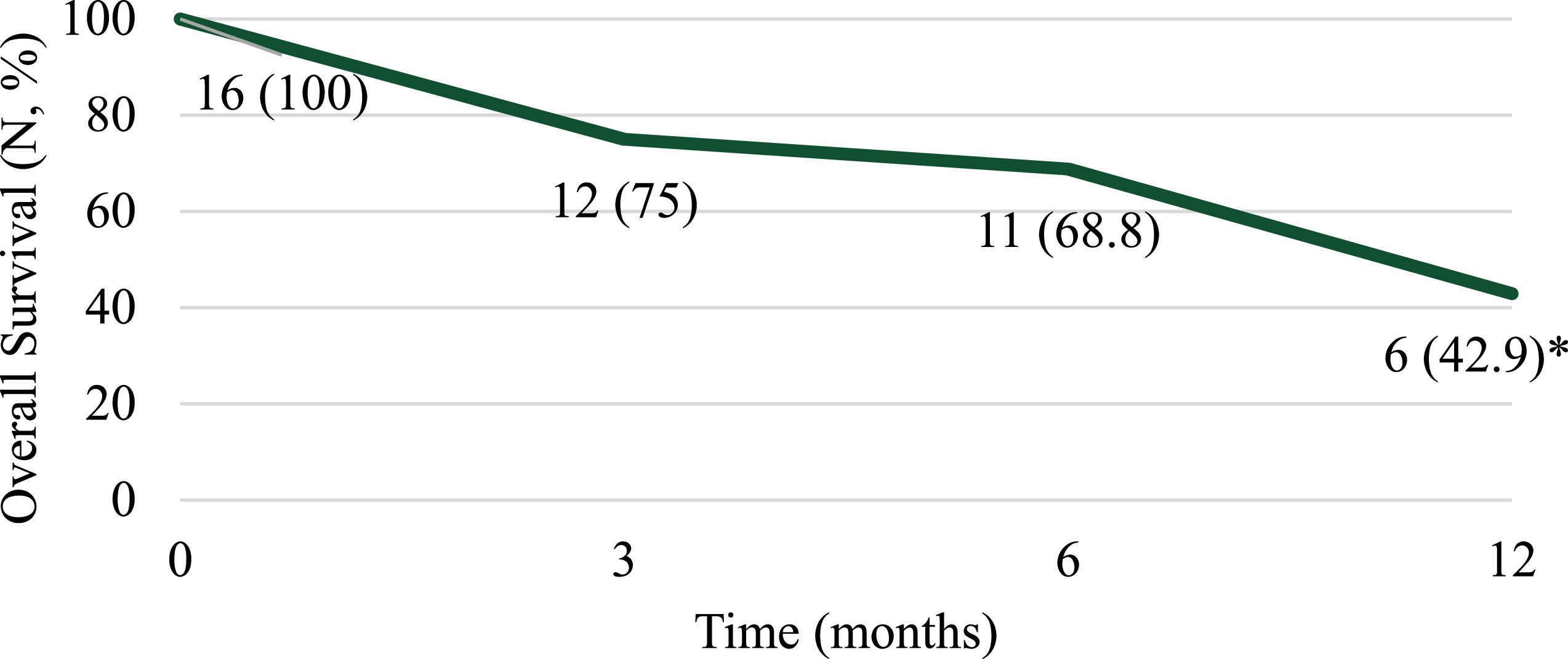
At 12 months post-transplant nine patients (69.2%) experienced progression of disease and eight patients (57.1%) died (Figures 1 and 2). Of note, 12-month response data were only available for 13 patients and 12-month overall survival data were only available for 14 patients. Patient mortality was attributed to disease progression for seven of the eight patients with sepsis and multiorgan failure being reported for the remaining patient. Among the four patients who completed a single transplant, three died due to disease progression and one patient remained relapse-free at 12 months post-transplant.
With upfront use of plerixafor at this center, 94% of patients achieved successful mobilization and 100% proceeded to initial transplant. Additionally, 69% of patients collected ≥ 4 × 106 CD34+ cells/Kg within a single apheresis session. Upfront use of plerixafor in this study did not adversely affect time to engraftment with median times to neutrophil and platelet engraftment of ten and 18 days, respectively, or long-term outcomes post-transplant. These outcomes are similar to those shown in previous studies of upfront plerixafor in non-Hodgkin's lymphoma and multiple myeloma that reported a significantly greater proportion of patients achieving successful mobilization with the addition of plerixafor plus G-CSF compared to placebo plus G-CSF.18-20 In a retrospective review of 50 patients with multiple myeloma, 25 received G-CSF alone as an upfront mobilization therapy and 25 received plerixafor 0.24 mg/Kg/day for up to four doses in addition to G-CSF.20 Plerixafor use resulted in fewer G-CSF doses required for stem cell collection, less apheresis sessions and quicker neutrophil recovery post-transplant which translated to a lower cost for G-CSF per patient for both stem cell collection and stem cell recovery as well as a lower apheresis cost per patient.20 Upfront plerixafor could be economically beneficial with the potential to reduce mobilization failures and decrease the number of apheresis days required for adequate stem cell collection. Of note, among the four patients excluded from this study that did not receive plerixafor during mobilization, 50% (n = 2) required only one day of collection and 50% (n = 2) required three days of collection. Based on the small sample size and no clear trend this should be evaluated in a larger prospective trial.
An alternative method of including plerixafor upfront is utilizing a risk-adapted approach based on peripheral blood CD34+ cell counts at baseline. This would target only those classified as poor mobilizers and are at risk of mobilization failure, which translates to nearly 30% of GCT patients.8 O'Hara et al. reported a case series describing the use of adjunct plerixafor in an attempt to prevent mobilization failure of nine patients with GCTs determined to be poor mobilizers.16 Following four days of G-CSF, plerixafor was initiated in all nine patients who collected less than 1.5 × 106 CD34+ cells/Kg after the first apheresis. Following administration of plerixafor, 100% of patients successfully collected CD34+ cells in a median of two days.16 A pre-post study conducted by Storch et al. evaluated the efficacy of a new mobilization algorithm that utilizes the Day 4 peripheral blood CD34 count to determine the need for plerixafor (n = 26) compared to a previous algorithm using the hematopoietic progenitor cell (HPC) value on Day 5 of mobilization to determine the need for plerixafor (n = 24).17 Utilization of the Day 4 peripheral blood CD34 count resulted in significantly fewer days of apheresis collection (1.25 days vs. 2.42 days; p-value = 0.001) without any significant change in the total number of CD34+ cells collected.17 Incorporating an adaptive mobilization model allows plerixafor to be incorporated in patients at risk of failing mobilization rather than upfront use in all patients or following low apheresis yields. However, currently there is no consensus on the optimal threshold of CD34+ cell counts that require the addition of plerixafor and this cannot be directly extrapolated from other patient populations that have been studied thus far. If proven to maintain fewer apheresis days and reduce re-mobilization attempts while also reducing drug costs and healthcare costs, a risk-adapted strategy could be the preferred strategy. This highlights the need for future studies to incorporate baseline CD34+ peripheral blood cell counts into a risk-adapted approach specifically in GCT patients.
A third strategy that has been studied is the inclusion of plerixafor after a failed mobilization attempt in GCT patients. In a retrospective analysis of 21 patients with GCTs who were remobilized with plerixafor plus G-CSF after failing initial mobilization, 43% went on to successfully collect ≥ 4 × 106 CD34+ cells/Kg in a median of three days.15 Sixteen (76%) of the patients proceeded to transplant and 38% received tandem transplants.15 This strategy is an option; however, it does not take into account the cost of mobilization attempts and delays or inability to proceed to HSCT. It has been reported that the cost of mobilization can account for 17-52% of total transplantation costs.21 The majority of these patients only required one day of apheresis collection with high enough yields sufficient for both planned transplants. Previous studies have reported an average of two days of apheresis when plerixafor was used following a risk-adapted approach, or three days when used for remobilization. Overall, out of the three strategies for including plerixafor as part of the mobilization strategy this study utilized upfront use to decrease delays to curative therapy, improve collection efficiency and decrease apheresis days. However, this should be confirmed as the preferred strategy in larger, prospective trials.
As for ease of administration, plerixafor is recommended to be administered approximately 11 hours prior to the initiation of apheresis.9 In this study, all patients received a single dose of plerixafor with an average time from plerixafor administration to start of apheresis session of 15.32 hours (range: 12.28-18.38). A previous single-center, retrospective analysis evaluating plerixafor administration at approximately 15 hours prior to apheresis also failed to find negative consequences associated with longer intervals between plerixafor and apheresis initiation22,23 Overall plerixafor is well tolerated with <2% of patients reporting adverse effects.24 In this retrospective study, no patients reported adverse events, such as arthralgia, diarrhea or nausea, related to plerixafor. Use of plerixafor did not adversely affect time to engraftment as evidenced by neutrophil and platelet engraftment times consistent with those historically reported.24 All patients demonstrated sustained engraftment with no transfusions or growth factor support required beyond 90 days post-transplant.
At 12 months post-transplant, 69.2% of patients relapsed with overall survival amongst all patients being 42.9%, which is similar to historical outcomes. Lorch et al. found 47% of patients who underwent auto-HSCT relapsed within 12 months of transplant.4 Reported 5-year overall survival ranges from 89% in a very low-risk group to 27% in a very high-risk group.6 Among the 16 patients reviewed, no transplant-related mortalities occurred. Follow-up data at 6 and 12 months post-transplant were not available for three patients (18.8%) as one patient was lost to follow-up and two had not reached that time point at the time of data collection. While the outcomes reported here are comparable to those previously reported for GCT patients undergoing auto-HSCT, they were only available for a small proportion of patients. In general, we do not feel the use of plerixafor upfront affected long-term outcomes in these patients but longer term follow-up studies are needed to confirm this and may potentially find a benefit with an earlier time to HSCT. The absence of certain data may limit the applicability of this study, including peripheral blood CD34+ count during mobilization, International Germ-Cell Cancer Collaborative Group (IGCCCG) risk status at diagnosis and long-term follow-up data for 18.8% of the patients.
ConclusionThe data described in this study demonstrate efficacy with the use of upfront plerixafor for mobilization of CD34+ cells in patients with GCTs. All but one patient (93.8%) met the predefined criteria for successful collection with upfront plerixafor use and all patients completed an initial transplant. Twelve patients (75%) completed tandem transplant; among the four patients who did not complete tandem transplant, an inadequate stem-cell count was not an attributable cause.
The significantly higher amount of CD34+ cells collected over less apheresis days demonstrated the clinical utility of plerixafor upfront and its potential to facilitate more efficient cell mobilization in patients with relapsed/refractory GCTs.
Author contributionsJaimie Cohen, Shreya Shah, Sila Shalhoub, Katrina Piedra, Cara Benjamin and Denise Pereira designed research and completed literature research, analyzed and interpreted the date, and wrote the manuscript. Jaimie Cohen performed data collection. All authors critically reviewed, provided edits, and approved the final manuscript.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.