Autologous hematopoietic stem cell transplantation (Auto-HSCT) is widely used in the treatment of patients with hematological neoplasms. Since these cells circulate in small quantities in the periphery, the use of regimens that promote their mobilization is essential. In this study, we retrospectively evaluated the efficacy and safety of using intermediate doses of cytarabine (1.6 g/m²) + filgrastim (10 mcg/kg/day) in the mobilization of stem cells in 157 patients treated by the Unified Health System at the Hematology and Bone Marrow Transplant Service of the Hospital Real Português de Beneficência, in Recife, Pernambuco. The sample included patients with multiple myeloma (MM) (58.6 %), lymphomas (29.9 %), and other neoplasms (11.5 %). The target of 2.0 × 10 6 CD34+ cells/kg was achieved by 148 (94.3 %) patients, in most cases (84.1 %) in a single apheresis and the median number of cells collected was 9.5 × 10 6 CD34+ cells/kg. No episode of febrile neutropenia was observed, however, 79 patients (50.3 %) required platelet transfusion (no cases attributed to bleeding). The median engraftment time was 11 days. Given these results, we suggest that the use of intermediate doses of cytarabine, combined with filgrastim, is safe and effective in mobilizing hematopoietic stem cells (HSCs).
Autologous Hematopoietic Stem Cell Transplantation (auto-HSCT) is an emergent strategy in the treatment of patients with lymphoid malignancies, such as multiple myeloma (MM) and Lymphomas.1-3 Currently, peripheral blood has largely replaced bone marrow as the major source of stem cells for auto-HSCT, due to a greater collection of CD 34+ cells for transplantation, shorter engraftment time and lower costs.2,4,5
Hematopoietic stem cells (HSCs) usually circulate in small numbers in peripheral blood. Therefore, the collection of sufficient autologous HSCs relies on the efficient mobilization of these cells from their bone marrow niche into circulation. The minimal safe number of CD 34+ cells required to ensure a successful multi-lineage engraftment after transplantation is considered to be 2 × 106 CD34+ cells/kg, with the optimal value being ≥ 5 × 106 CD34+ cells/kg, which is associated with a better post-transplant response (shorter engraftment time and lower need for transfusions).2,3,6
The granulocyte colony-stimulating factor (G-CSF) is the most commonly used mobilization agent at several centers. However, G-CSF alone fails to yield adequate CD34+ cells in approximately 5 to 30 % of patients with MM or lymphoma; therefore, its use is very limited to patients with a low risk of mobilization failure.7 Thus, the HSCs mobilization ability of other agents (alone or in association) has been widely investigated. One option is based on G-CSF in combination with chemotherapy, especially cyclophosphamide, which could improve the CD34+ cell yield, but at the expense of increased toxicity.6,8
In addition to this, a significant proportion of patients still fail to mobilize HSCs, especially those with predictors of poor mobilization, as suggested by the Italian Bone Marrow Transplant Group: previous mobilization failure, prior exposure to radiation and chemotherapy, an advanced disease with low bone marrow reserve (cellularity < 30 %), and age over 65 9,10
In recent years, several reports indicated that intermediate-dose cytarabine (ID-Ara-C) may be particularly safe and effective as a mobilization protocol. In a single-center study, ID-Ara-C + G-CSF used as a first-line mobilization was found more effective than cyclophosphamide + G-CSF.9 Other studies conducted with patients with MM and lymphomas also showed the high efficacy of using Ara-C as a first-line or second-line regimen.11,12
This study aimed to assess the efficacy and safety of using cytarabine to mobilize CTH in patients with lymphoid system neoplasms at a referral hospital in northeastern Brazil.
MethodologyPatientsWe analyzed the results of 157 consecutive patients with lymphoid malignancies, who underwent hematopoietic stem cell mobilization with ID-Ara-C + G-CSF as an outpatient regimen, between January 2014 and January 2016 at the Hematology and Bone Marrow Transplant Service of the Hospital Real Português de Beneficência, in Recife, Pernambuco. Only one auto-HSCT was planned for each patient. None of the patients had received an auto-HSCT before.
Mobilization and leukapheresis regimenAra-C was administered as a 2-hour IV infusion at a dose of 0.4 g/m², twice daily on days 1 and 2. G-CSF (10 ug/kg/day) was started on day 5 and continued until the last leukapheresis. Platelet transfusion was indicated when levels dropped below 20,000/mm³ or 50,000/mm³, when bleeding was present and/or on the day of apheresis. Packed RBCs were administered when the hemoglobin was lower than 8.0 g/dL. The patients were also monitored for the presentation of episodes of febrile neutropenia.
Peripheral CD34+ cells were not counted due to the unavailability of a flow cytometer in the service. Leukapheresis was therefore performed when the total leukometry reached 5000 cells/mm³. The procedure was performed by processing 4 to 6 blood volumes, using the COBE® Spectra machine, to collect 2.0 × 106 CD34+ cells/kg.
The measurement of collected CD34+ cells was performed at a central laboratory. Mobilization failure was considered when the collection was lower than 0.7 × 106 CD34+ cells/kg during the first apheresis or when it was lower than 2.0 × 106 CD34+ cells/kg after two aphereses.
Data collectionWe accessed patient records available at the Hematology and Bone Marrow Transplant Service of the Hospital Real Português de Beneficência, in Recife, Pernambuco. The collection was approved by the Research Ethics Committee of the Medical Sciences Center of the Universidade Federal da Paraíba.
The variables were divided into epidemiological, efficacy and safety. The epidemiological variables were gender (female and male), age (in years), and diagnosis (myeloma, lymphomas and others); the efficacy variables were the number of CD34+ cells collected, number of aphereses performed and time of engraftment (in days) and, finally, the safety variables comprised the presence of episodes of febrile neutropenia during mobilization and the need to perform transfusions.
Statistical analysisThe variables are presented in a descriptive analysis form, with continuous variables being expressed as a median with an interquartile interval, and the categorical variables, in the form of absolute and relative frequencies.
Efficacy and safety variables were compared between the different subgroups created according to their baseline characteristics (gender, age and diagnosis). The Mann-Whitney and Kruskal-Wallis tests were used to compare quantitative dependent variables and the exact Fischer test, for comparison, when the dependent variables were categorical. The Spearman correlation test was used to assess whether there was a correlation between age and the number of CD 34+ cells collected and this, with the engraftment time.
All analyses were performed using the R© program version 4.1.3. The significance level used was 0.05.
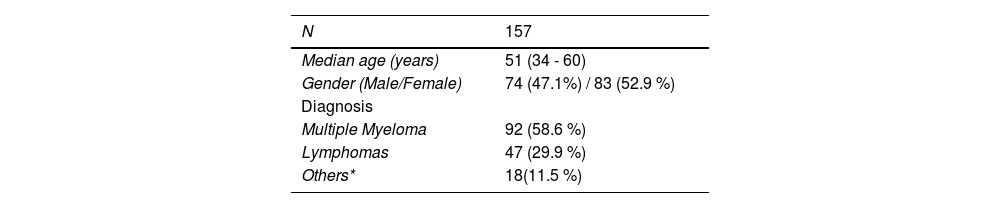
ResultsPopulationOur sample comprised a total of 157 patients who underwent hematopoietic stem cell mobilization with ID-Ara-C + G-CSF at the Hematology and Bone Marrow Transplant Service of the Hospital Real Português de Beneficência, all through the Sistema Único de Saúde (SUS), in Recife, Pernambuco. The main patient characteristics are summarized in Table 1. The majority of patients had Multiple Myeloma (58.6 %), followed by patients with lymphomas (29.9 %) and other hematological malignancies (11.5 %) (Table 1).
Baseline characteristics.
| N | 157 |
|---|---|
| Median age (years) | 51 (34 - 60) |
| Gender (Male/Female) | 74 (47.1%) / 83 (52.9 %) |
| Diagnosis | |
| Multiple Myeloma | 92 (58.6 %) |
| Lymphomas | 47 (29.9 %) |
| Others* | 18(11.5 %) |
Among the MM patients, 60 (65.2 %) were in very good partial response, 25 (27.2 %), in complete response, and the remaining, in partial response. None of the MM patients had used lenalidomide as induction therapy and the majority, totaling 75 (81.5 %), were treated with cyclophosphamide in association with thalidomide and dexamethasone and 12 (13 %) received radiotherapy in the pelvic bone.
Hodgkin's lymphoma was the most common lymphoma in this cohort, with 29 patients, corresponding to 61.7 % of the lymphomas. A total of 36 patients with lymphoma (76.6 %) were in their second remission and 18 (38.3 %) received radiation therapy.
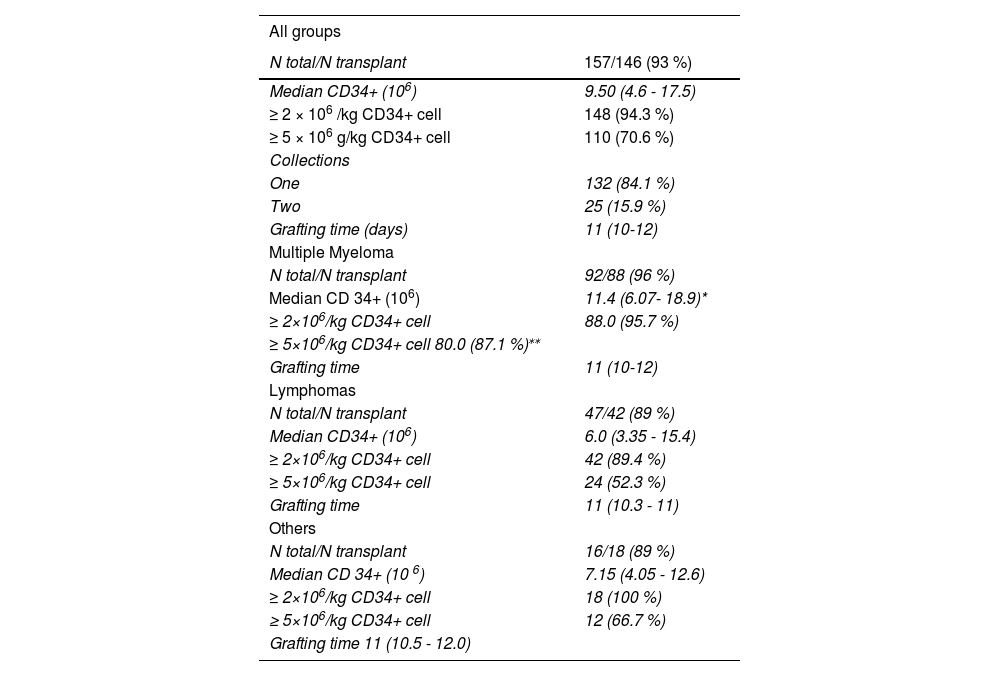
Effectiveness in hematopoietic stem cell collection and transplantationA total of 146 (93 %) patients underwent auto-HSCT after collecting CD34+ cells. Among the 11 patients who did not proceed to transplant, 9 resulted from failure in the mobilization, while the other 2 developed complications inherent to the underlying disease. A total of 148 patients (94.3 %) reached the target CD34+ cell dose (2.0 × 106 CD34+ cells/kg) and 110 (70.6 %) were able to achieve values greater than or equal to 5.0 × 106 CD34+ cells/kg (Table 2).
Effectiveness in the collection and transplantation of HSCs.
| All groups | |
|---|---|
| N total/N transplant | 157/146 (93 %) |
| Median CD34+ (106) | 9.50 (4.6 - 17.5) |
| ≥ 2 × 106 /kg CD34+ cell | 148 (94.3 %) |
| ≥ 5 × 106 g/kg CD34+ cell | 110 (70.6 %) |
| Collections | |
| One | 132 (84.1 %) |
| Two | 25 (15.9 %) |
| Grafting time (days) | 11 (10-12) |
| Multiple Myeloma | |
| N total/N transplant | 92/88 (96 %) |
| Median CD 34+ (106) | 11.4 (6.07- 18.9)* |
| ≥ 2×106/kg CD34+ cell | 88.0 (95.7 %) |
| ≥ 5×106/kg CD34+ cell 80.0 (87.1 %)⁎⁎ | |
| Grafting time | 11 (10-12) |
| Lymphomas | |
| N total/N transplant | 47/42 (89 %) |
| Median CD34+ (106) | 6.0 (3.35 - 15.4) |
| ≥ 2×106/kg CD34+ cell | 42 (89.4 %) |
| ≥ 5×106/kg CD34+ cell | 24 (52.3 %) |
| Grafting time | 11 (10.3 - 11) |
| Others | |
| N total/N transplant | 16/18 (89 %) |
| Median CD 34+ (10 6) | 7.15 (4.05 - 12.6) |
| ≥ 2×106/kg CD34+ cell | 18 (100 %) |
| ≥ 5×106/kg CD34+ cell | 12 (66.7 %) |
| Grafting time 11 (10.5 - 12.0) |
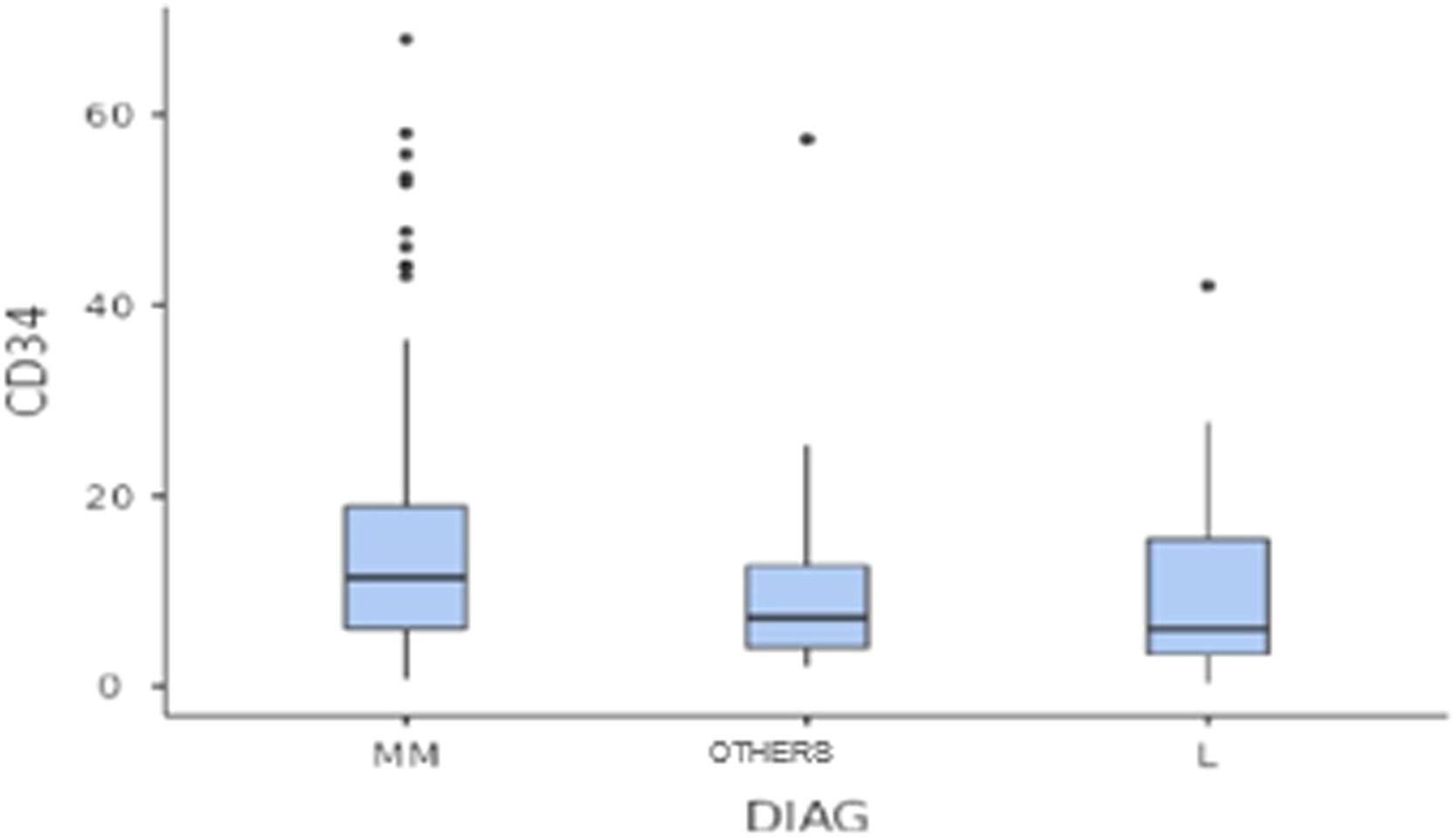
The median number of CD34+ cells collected was 9.5 × 106 cells/kg. However, patients with multiple myeloma had a higher median number of CD 34+ cells collected than the group of patients with lymphoma (11.4 × 106vs. 6.0 × 106, respectively; p < 0.05) (Figure 1).
The MM group also reached a higher proportion of individuals from whom it was possible to collect at least 5.0 × 106 CD34+ cells/kg, compared to the other groups (87.1% vs. 52.3% vs. 66.7 %, respectively MM, L, OTHERS; p < 0.05).
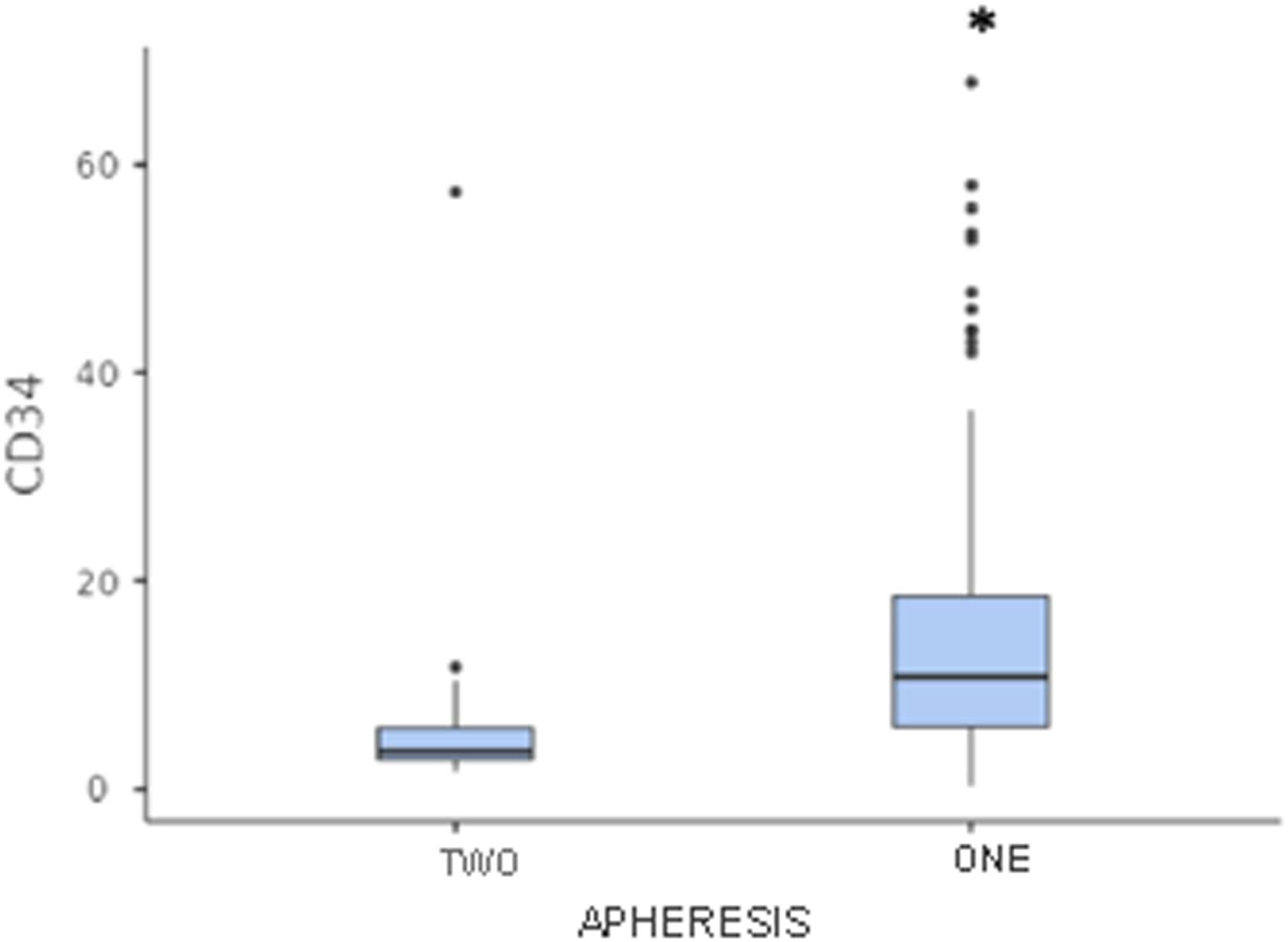
A single apheresis was sufficient to collect adequate numbers of CD34+ cells in 84.1 % (132) and the median engraftment time was 11 days. Only 25 patients (15.9 %) underwent two aphereses.
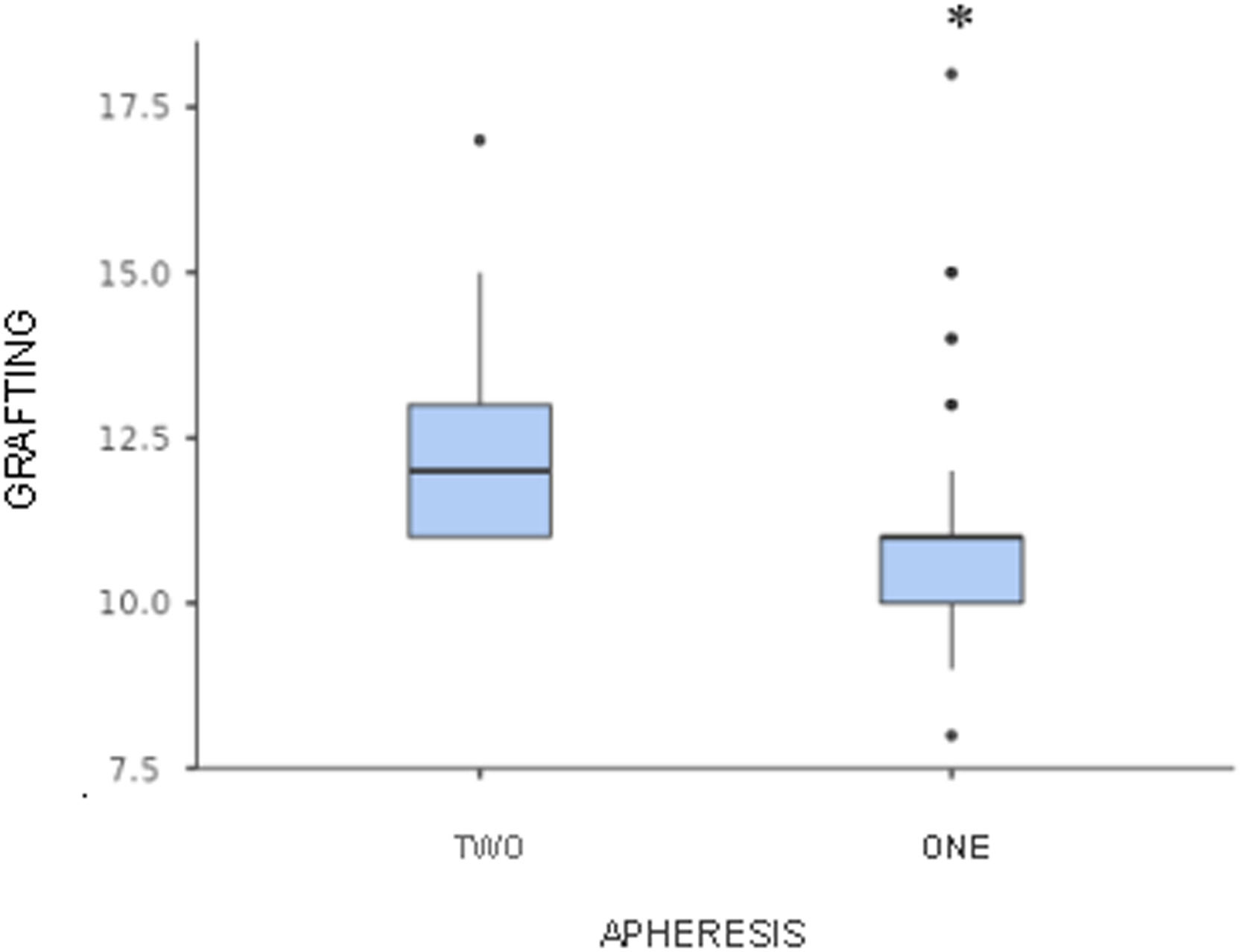
Patients who underwent only one apheresis had a higher median number of CD34+ cells collected and a shorter engraftment time, compared to patients who required two aphereses (median number of CD34+ cells collected: 10.8 × 106 cells/kg vs. 3.6 × 106 cells/kg, respectively; median grafting time: 11 days vs. 12 days, respectively; p < 0.05) (Figures 2 and 3).
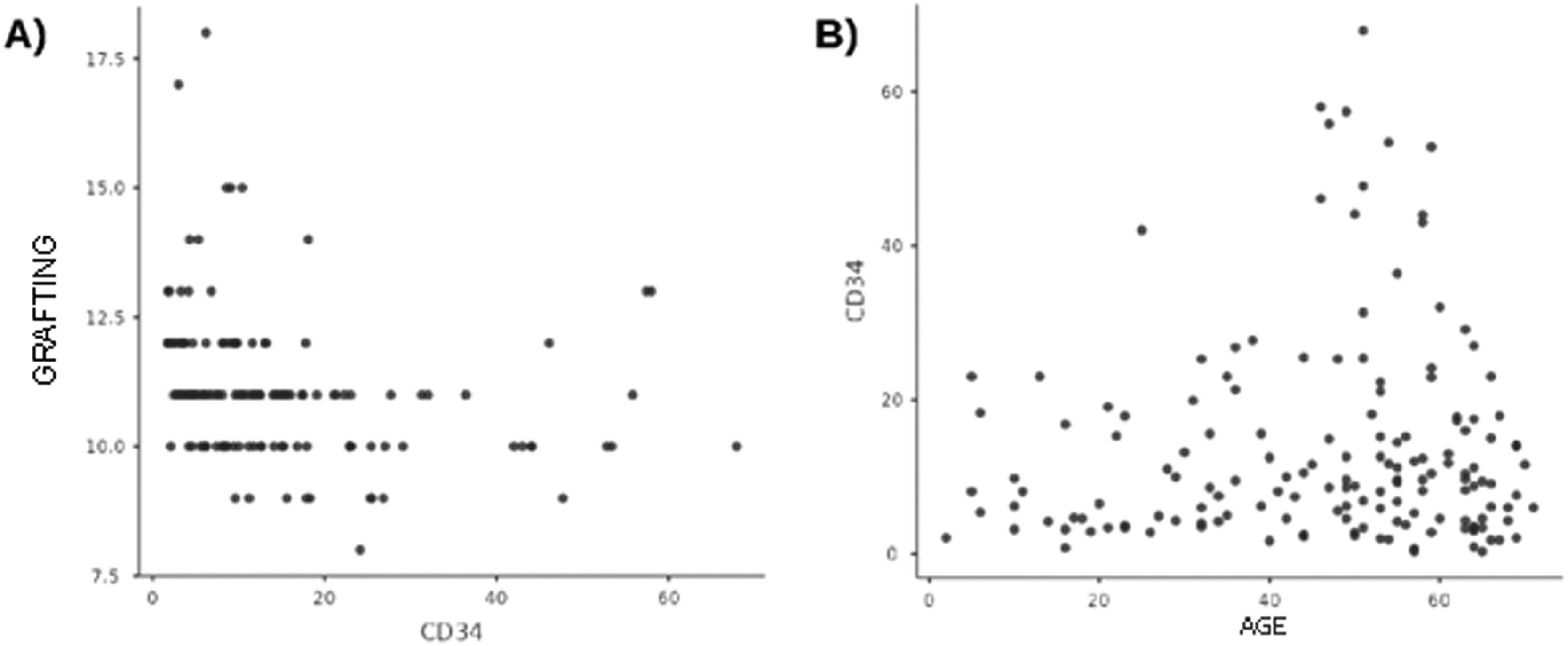
There was no correlation between age and CD34+ cells collected, nor between the latter and the time of engraftment (Figure 4).
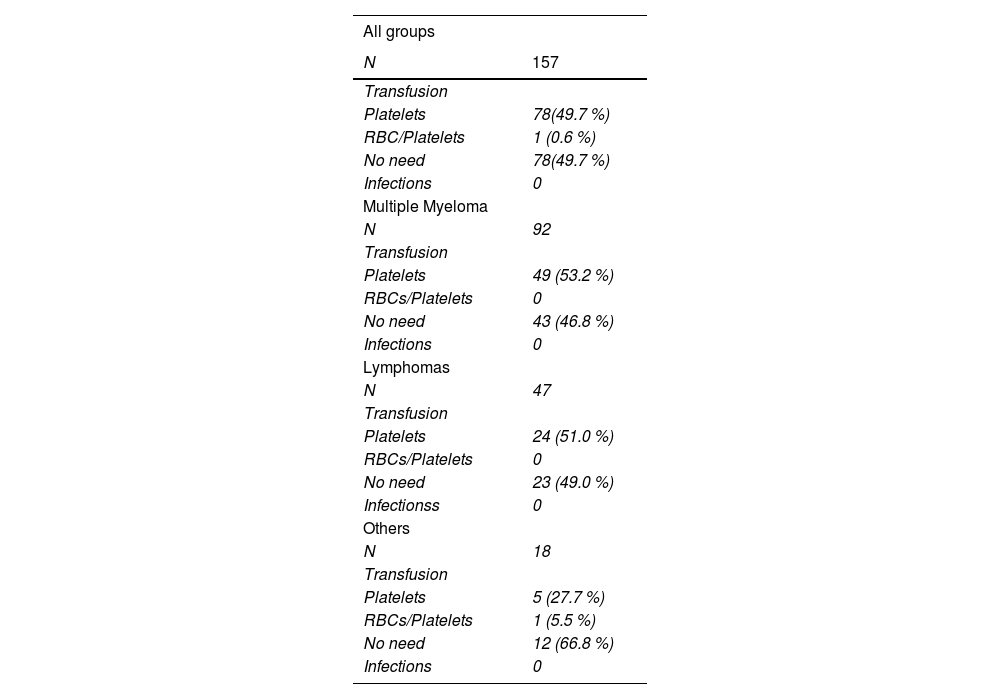
Safety in the mobilization of hematopoietic stem cellsNo episode of febrile neutropenia was observed. As for the need for blood component transplants, 78 patients (49.7 %) did not require any type of transfusion support, while another 78 (49.7 %) needed platelet transfusion, but none due to bleeding. Finally, only one patient needed to use packed RBCs + platelet transfusion (Table 3). No patient had to be hospitalized during the mobilization process.
Safety when mobilizing HSCs.
Today, it is believed that the choice of the ideal mobilization regimen should be personalized, taking into account both the presence of risk factors for poor mobilization, as well as logistical and cost issues. Thus, the strategies used tend to vary within the various transplant centers around the world. For patients with a low risk of mobilization failure and who are planning to undergo a single transplant, the use of G-CSF alone is recommended. On the other hand, a patient with strong predictors to fail in mobilization or who requires a larger collection of CD34+ cells for a double transplant, for example, should benefit from the use of G-CSF associated with chemotherapeutic drugs. Another mobilization strategy involves the use of plerixafor, an antagonist of the interaction of stroma-derived factor 1 (SDF-1) with the CXCR4 receptor, which retains stem cells in the bone marrow. Although it has proven to be a good alternative, in addition to being a lifesaver in patients with failed previous regimens, its availability as a first-line approach is limited in many countries due to its high cost.2,8,13
Among the chemotherapeutic agents, cyclophosphamide (Cy) is the most studied and used for chemo-mobilization regimens in patients with lymphoid malignancies. Narayanasami et al. demonstrated in a randomized clinical study that the addition of Cy at a dose of 5 g/m² to G-CSF was capable of inducing a higher collection of CD 34+ cells than that performed using G-CSF alone (median, 7.2 × 106 cells/kg versus 2.5 × 106 cells/kg, respectively), but this was not reflected in other outcomes, such as the number of aphereses needed to reach the target and the grafting time, both of which were similar in the two test groups.14 In a meta-analysis involving prospective and retrospective studies, it was concluded that the association between G-CSF and Cy showed superiority in terms of the number of CD 34+ cells collected in patients with MM. However, a greater need for hospitalizations and a greater number of febrile episodes were also observed with the use of Cy.15,16
Recently, cytarabine (Ara-C) has been extensively studied at some centers as an alternative to the use of Cy. One of the main findings of a recent meta-analysis produced by Luo et al. was the superiority of the use of intermediate doses of Ara-C + G-CSF over Cy + G-CSF to mobilize HSCs in patients with MM.8 In a retrospective study, which evaluated the use of Ara-C compared to the use of Cy, an increase in the efficacy of the collection of HSCs was observed in the group of patients mobilized with Ara-C, evidenced by the peak of peripherical CD34+ cells before apheresis, which was almost 4 times the value observed in the group with Cy (median of 120 cells/µl vs. 33 cells/µl, respectively, p < 33 cells/µl, respectively, p < 0.05).9 Other studies, including new clinical trials and retrospective studies, demonstrated the superiority of the use of Ara-C, compared to regimens with G-CSF alone or combined with other chemotherapeutic agents, especially in patients with MM.1,14,17–20
Our results suggest the efficacy and safety of using cytarabine to mobilize hematopoietic stem cells in patients with lymphoid malignancies. The median number of CD34+ cells observed in our cohort was 9.5 × 106 cells/kg, which was significantly higher for patients with MM, compared to patients with lymphomas (median, 11.4 × 106 cells/kg vs. 6.0 × 106 cells/kg, respectively, p < 0.05). The target of 2.0 × 106 cells/kg was reached in 94.3 % of all patients, most of the time in a single apheresis (84.1 %), without measuring the number of circulating CD34+ cells, as a flow cytometer was unavailable. Of the patients in the myeloma group, who can benefit from a double transplant, 80 patients (87.1 %) achieved values greater than, or equal to, 5.0 × 106 CD34+/ kg, while of the patients in the lymphoma group, 42 (89.4 %) reached the target of 2.0 × 106 cells/kg, sufficient for a single transplantation. Giebel et al., in one of the first studies using cytarabine in patients with lymphoid malignancies (including poor mobilizers), had already observed good results using Ara-C for mobilization, with 97 % of the patients reaching the target of 2.0 × 106 cells/kg (91 % in the first apheresis).9 They were also able to show a better response to the regimen in patients with MM, which has also been demonstrated in several other studies recently published by Jelinek et al., Bogucka-Fedorczuk et al., Bogucka-Fedorczuk et al. and Czer et al..17,18,20 Giebel et al., in 2016, also found good efficacy in the use of Ara-C in patients with lymphomas, with 41 (82 %) of the patients achieving the target of CD34+ cells in a single apheresis.1
A Brazilian cohort, recently published by Callera et al., evaluated the use of intermediate doses of cytarabine for the mobilization of HSCs in 81 patients. These were separated into different groups (A and B), one with patients newly diagnosed with MM (A) and the other with patients with lymphomas, non-promyelocytic AML and germ tumors previously treated with at least two different chemotherapy regimens associated or not with radiotherapy, in addition to patients with MM who failed the first auto-HSCT (B). The aimed result for the patients in group A was to collect 5.0 × 106 CD34+ cells/kg, which was achieved by 98 % of the patients, in most cases already in the first apheresis (92 %). For Group B, on the other hand, the aimed result was to collect 2.0 × 106 CD34+ cells/kg and, as expected, a lower percentage of patients were able to achieve it (88 %).21
Regarding safety variables, our study focused on identifying episodes of febrile neutropenia and transfusion needs (red blood cells, platelets and red cells/platelets). Episodes of neutropenia are well described with the use of cyclophosphamide, as observed by Giebel et al. and Jelinek et al., who showed more frequent episodes of grade 4 neutropenia, in the range of 70 - 73 %, in patients who had used the Cy, while in patients mobilized with Ara-C, this finding was less common (20 - 36 %).9,18 In this retrospective cohort, no infectious episode was documented, and regarding transfusion needs, 79 (50.3 %) of the patients needed to receive platelet transfusion, but none of them were due to bleeding episodes. Thrombocytopenia is a common finding when mobilized with cytarabine. Several studies, such as those conducted by Jelinek et al. (2019), and Bogucka-Fedorczuk et al. (2020), showed a greater need for platelet transfusions in patients mobilized with Ara-C, with relative frequencies ranging from 32.6 to 48 %, and the opposite was observed in patients mobilized with cyclophosphamide, with frequencies of 7 to 10 %.18,20 Callera et al., in their cohort that evaluated the use of cytarabine, did not document any episodes of febrile neutropenia, however, 45.6 % of the patients required platelet transfusion.21
This study has the limitation of being susceptible to information biases, as the quality of the data collected is completely dependent on how they are described in medical records by the various professionals involved in patient care. Another limitation is the absence of an active comparator arm, either a control group or another intervention. However, this is the study with the largest sample of enrolled patients (157 patients), submitted to the same mobilization regimen. It is also worth mentioning that the patients involved had all the care provided by the SUS and the samples were collected without performing a peripheral CD34+ cell count due to the unavailability of a flow cytometer, which did not influence the effectiveness of the collection, as the target was achieved by 94.3 % of the cases.
In short, we were able to observe that the use of intermediate doses of cytarabine, associated with G-CSF, allowed for adequate collection of CTH, in most cases in a single apheresis, without prior counting of circulating CD34+ cells, demonstrating the viability of practicing this mobilization regimen in SUS services that do not have the technological apparatus of the flow cytometer. Thrombocytopenia was the main complication, reflected by the need for transfusion. New studies still need to be developed, especially randomized clinical trials comparing chemo-mobilization regimens currently in use and, if possible, cost-effectiveness studies too.