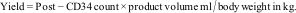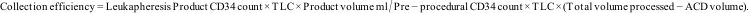To study the efficacy and safety of single large volume leukapheresis by using generic G-CSF or G-CSF plus Plerixafor in achieving adequate stem cell yield and various factors influencing thereof in newly diagnosed multiple myeloma patients undergoing autologous stem cell transplant .
MethodThis prospective study was undertaken among 55 newly diagnosed multiple myeloma patients undergoing autologous stem cell transplant and aged between 18 and 75 years. Mobilization and harvesting of stem cells were performed by using GCSF or GCSF plus Plerixafor and large volume leukapheresis, respectively. A stem cell yield of ≥2×106kg–1 and the number of apheresis procedures were primary efficacy endpoints, while the ideal stem cells yield >5×106kg–1, the engraftment day and D100 response/graft sustainability were secondary endpoints.
ResultThe primary endpoint was achieved in all cases in both the groups by using a single LVL leukapheresis procedure. Fulfillment of all the secondary endpoints was satisfactory and comparable in both the groups. Age, pre-apheresis CD34+ count and number of interruptions during the LVL were significant factors influencing the stem cell yield (p<0.05). Adverse drug reactions during the apheresis and post-ASCT period were manageable.
ConclusionThe LVL is safe and cost-effective in attaining a minimum of CD34+ cells in a single procedure with manageable adverse reactions. Judicious intervention during the procedure may be helpful in ensuring the adequate yield.
High-dose chemotherapy (HDT), followed by autologous stem cell transplantation (ASCT), has remained the standard of care for multiple myeloma (MM) for the last three decades, as it gives a better survival advantage, compared to chemotherapy (CT) alone.1,2 Adequate CD34+ stem cell mobilization is an important pre-requisite step for the successful outcome of ASCT. However, approximately 20% of the patients with MM are poor mobilizers, either with cytokines alone or cytokines and chemotherapy.3 Repeated CT, prior radiotherapy, old age, advanced disease with extensive marrow involvement and altered bone marrow milieu are known limiting factors for successful mobilization due to compromised stem cell (SC) reserve. Multiple mobilization attempts in these patients is not only a cumbersome procedure, but also increases the transplantation cost, which may be a limiting factor for many patients in a developing country.4 Approval of Plerixafor for stem cell mobilization is a landmark development that has enhanced the success rate of adequate stem cell yield.5,6 It is the selective chemokine receptor CXCR4 antagonist facilitating the mobilization of stem cells from bone marrow to peripheral blood.7,8 Availability of generic Plerixafor with low cost has improved the affordability. This Institution has successfully mobilized stem cells with adequate yield in a single setting apheresis and transplanted them into elderly patients with MM (>65 years) with the help of generic Plerixafor for first time in India.9 Conventional hematopoietic cells collected by apheresis (HPC(A)) typically involves the processing of two to three blood volumes of the patient/donor, whereas large volume leukapheresis (LVL) processes three to six total blood volumes (up to 20–36L of blood).10,11 The LVL procedure takes more time, entails a greater loss of platelets and increases the possibility of side effects, but is cost-effective in comparison with the conventional HPC(A) procedure. Thus, the LVL procedure may be affordable to most of the patients in resource-constrained settings.12,13 One Indian study has compared the extraction efficiency of the LVL procedure performed in a non-uniform cohort (MM, Hodgkin's disease, non-Hodgkin's lymphoma, germ cell tumor and amyloidosis) of patients undergoing an ASCT by using the granulocyte colony stimulating factor (G-CSF) alone.14 This was a retrospective analysis of factors influencing the stem cell yield, without any reporting on safety and post-ASCT parameters, such as engraftment or D+100 graft sustainability.
The present prospective study was undertaken with the following objectives:
- 1.
To determine the efficacy and safety of the LVL procedure in achieving adequate stem cell yield in newly diagnosed MM (NDMM) patients by using generic G-CSF, with or without Plerixafor.
- 2.
To investigate the various factors influencing the stem cell yield.
This prospective non-randomized study was undertaken in the Department of Clinical Hematology, SCB Medical College and Hospital, India, over the last 3 years (2014–17). Fifty-five consecutive NDMM (according to the International Myeloma Working Group Revised Diagnostic Criteria, 2014), qualified and willing to undergo ASCT at our center, were included in the study.15 The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the institutional ethics committee. All patients provided written informed consent.
Study populationInclusion criteriaPatients with NDMM between 18 to 75 years of age achieving satisfactory therapeutic response (≥very good partial response (VGPR), according to the revised uniform response criteria by the International myeloma working group, 2016), following three to six cycles of VTD (Velcade/bortezomib, thalidomide, dexamethasone), and willing to receive ASCT were included in the present study.16 Other essential criteria included were: performance status more than 70% (Karnofsky scale), satisfactory organ function (cardiac, hepatic, renal and pulmonary), negative viral markers (HBV, HCV, HIV, CMV, EBV and herpes). Women of child-bearing age group agreeing to submit consent for contraception were also included in the study. Comorbidities, such as hypertension and diabetes, were not considered as contraindications for inclusion.
Exclusion criteriaPatients were excluded if they had a creatinine clearance <30mL/min, aspartate transaminase (AST) ≥2 times the upper normal limit, cardiac ejection fraction ≤55% or abnormal pulmonary function test FEV1/FEV <70%. Patients who received 2 or more alkylating agents, a total dose of ≥200mg/m2 of prior melphalan as a part of CT, granulocyte colony-stimulating factor (G-CSF) within 14 days before the first dose of G-CSF for mobilization, failed previous hematopoietic stem cell collections or collection attempts or had prior radiation therapy, any comorbidities rendering them at high risk for treatment complications or acute infections, were excluded from the study. Female patients testing positive to a pregnancy test or lactating females were also excluded.
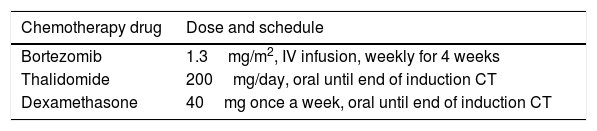
Treatment plan and transplantationAll of our patients were evaluated with detailed history, general and systemic examinations, hematologic and biochemical parameters, bone marrow study, serum protein electrophoresis and immunofixation, estimation of serum-free light chain (FLC) ratio, urine analysis and 24h urinary protein estimation and skeletal survey (X-ray and MRI), as per the standard protocol. All cases received uniform induction CT (3–6 cycles of bortezomib, thalidomide and dexamethasone and, depending on the response, each cycle was of a 4-week duration), as presented in Table 1, along with other supportive measures.
After achievement of a satisfactory response (≥VGPR), a detailed pretransplant evaluation was made in all cases, as per inclusion/exclusion criteria. Detailed counseling and other procedures were followed, as per the standard protocol.
Stem cell mobilizationAfter satisfactory recovery from the last induction CT (white blood count >2.5×109L–1, an absolute neutrophil count >1.5×109L–1 and a platelet count >100×109L–1), the G-CSF was administered subcutaneously once daily each morning (prior to harvest on days of apheresis until completion of harvest) at 8:00 a.m. at a dose of 10μg/kg/day from D1 to D5. The total leukocyte count (TLC) and CD34 count were evaluated on D4 at 10:00 a.m. by the fully automated cell counter Sysmex XT and BD FACSCanto™ II, respectively. On D4 the CD34 count was estimated from the peripheral blood 2h after the G-CSF dose. If the CD34 count was ≥10μL−1 on D4 and/or D5, stem cell apheresis was performed on D5 by the COBE spectra apheresis equipment. If the CD34 count was less than 10μL−1 on D4, Plerixafor (0.24mg/kg if creatinine clearance (Crcl) was ≥50mL/min or 0.16mg/kg if the Crcl was 30–49mL/min) was administered subcutaneously (11:00 p.m. on D4), followed by G-CSF at the usual dose at 8:00 a.m. on D5. The CD34 count was re-estimated on D5 at 10:00 a.m. by flow cytometer. If the CD34 count was ≥10μL−1, apheresis was immediately performed on same day. If the CD34 count on D5 was less than 10μL−1, another dose of Plerixafor was given at 11:00 p.m. on D5, followed by G-CSF and stem cell enumeration on D6, as mentioned earlier. If the CD34 count on D6 was ≥10μL−1, apheresis was performed. If the CD34 count on D6 was less than 10μL−1, apheresis was postponed. The stem cell apheresis procedure was repeated on the second consecutive day if the stem cell yield was <2×106 CD34+ cells/kg.
Apheresis procedureAll the LVL processes were performed with the COBE spectra cell separator, as per the manufacturer instructions, and the software version 7.0, which has a higher CD34 yield and better collection efficiency. The settings for LVL were adjusted to the mean yield of 5×106 CD34+ cells/kg (predicted value) with a low extracorporeal volume of 285mL, so that no blood component would be required during the procedure. The average anticoagulant citrate dextrose (ACD) solution to blood flow rate was 1:14. The vitals of the patient were monitored hourly. All of the patients were given prophylactic oral calcium tablets every 30min during the procedure. Stem cell collection by apheresis was conducted, as per the manufacturer's guidelines, and started after the mononuclear cell collection tubing matches with the provided colorigram. If there was any interruption of the procedure due to non-cooperation, bathroom urgency, rigor, blockage of access/return line, etc., or any other reason, the procedure was restarted by following standard operating procedures. One dedicated apheresis expert was always in vigilance to modify the plasma flow rate, as per the need to match the color of the collection line with the colorigram. After completion of the apheresis, the complete blood count (CBC), CD34 count estimation and microbiological culture of the product were made. The stem cell yield (actual value) was calculated by using the following formula:
The goal of the stem cell collection was 5×106 CD34+cells/kg, with a minimum dose of 2×106CD34+ cells/kg. The collected stem cells were stored at 2–8°C in a blood bank refrigerator and infused into the patient on the next day.
Conditioning regimenAll were conditioned with high dose melphalan (200mg/m2, 140mg/m2, if the glomerular filtration rate (GFR) were less than 50mL/min/1.73m2) at 9:00 p.m. on the day of the satisfactory stem cell yield by following standard procedures and supportive measures (hydration, antiemetic, ice suckling, etc.). Stem cells were infused intravenously (IV) on the next day at 12 noon. The G-CSF (5mg/kg/day) was administered from the fifth post-transplant day until engraftment. Platelet and red cell support were provided whenever necessary. Levofloxacin, acyclovir and fluconazole were used as infection prophylaxis, along with other supportive treatment.
Neutrophil engraftment was defined as the neutrophil count ≥0.5×109L–1 for 3 days or ≥1.0×109L–1 for 1 day. Platelet engraftment was defined as the platelet count ≥20×109L–1, without a transfusion for the preceding 7 days. Graft sustainability on D100 was defined as the maintenance of blood counts according to at least 2 of the following 3 criteria: a platelet count more than 50×109L–1, without transfusion for at least 2 weeks before the follow-up visit, a hemoglobin level ≥10g/dL, with no erythropoietin support or transfusions for at least 1 month before the follow-up visit, absolute neutrophil count >1×109L–1, with no G-CSF for at least 1 week before the follow-up visit. Patients were discharged 3 days after engraftment and were advised to undergo regular follow-up for evaluation. Subsequently, on D+100 all patients were evaluated for the status of MM (M protein, free light chains, serum protein, serum calcium, Beta 2 microglobulin and serum lactate dehydrogenase (LDH)) and graft sustainability, as mentioned earlier. From D+101, all of the patients received maintenance CT with either lenalidomide at 10mg/day or bortezomib at 1.3mg/m2 subcutaneously every 2 weeks until progression or unacceptable toxicities.
Efficacy and safety assessmentsThe primary efficacy endpoints were the number of patients achieving minimum adequate stem cell yield (2×106 CD34+ cells per kg) and number of apheresis processes achieving this. The secondary efficacy endpoints were the number of patients achieving ideal stem cell yield (≥5×106 CD34+cells/kg), the engraftment day, safety of the procedure, well-being of the patients during apheresis and graft sustainability on D100.
The safety of the LVL was monitored by observing vital signs, clinical/laboratory parameters and adverse events/reactions (ADRs) during the procedure. Adverse events occurring up to 100 days after the ASCT were documented.
StatisticsCategorical data were summarized in frequency tables by the number and percentage of patients falling into each category. Continuous data were summarized using descriptive statistics, including the number of observations, mean, standard deviation, median and minimum and maximum values. A linear regression analysis was performed to evaluate the impact of patient/donor age, gender, BMI (body mass index), BSA (body surface area in m2), hemoglobin (Hb), leukocyte count, platelet count, Karnofsky PS, pre-apheresis CD34 count, type of pretransplant myeloma response (stringent complete response (sCR) vs. VGPR) and interruption of the LVL procedure (for reasons, such as non-cooperation, bathroom urgency, rigor, blockage of access or return line, etc.) as independent variables, with stem cell yield as the dependant variable. A p value of < 0.05 was considered statistically significant. All statistical analyses and calculations were made using the trial version of SPSS version 19.
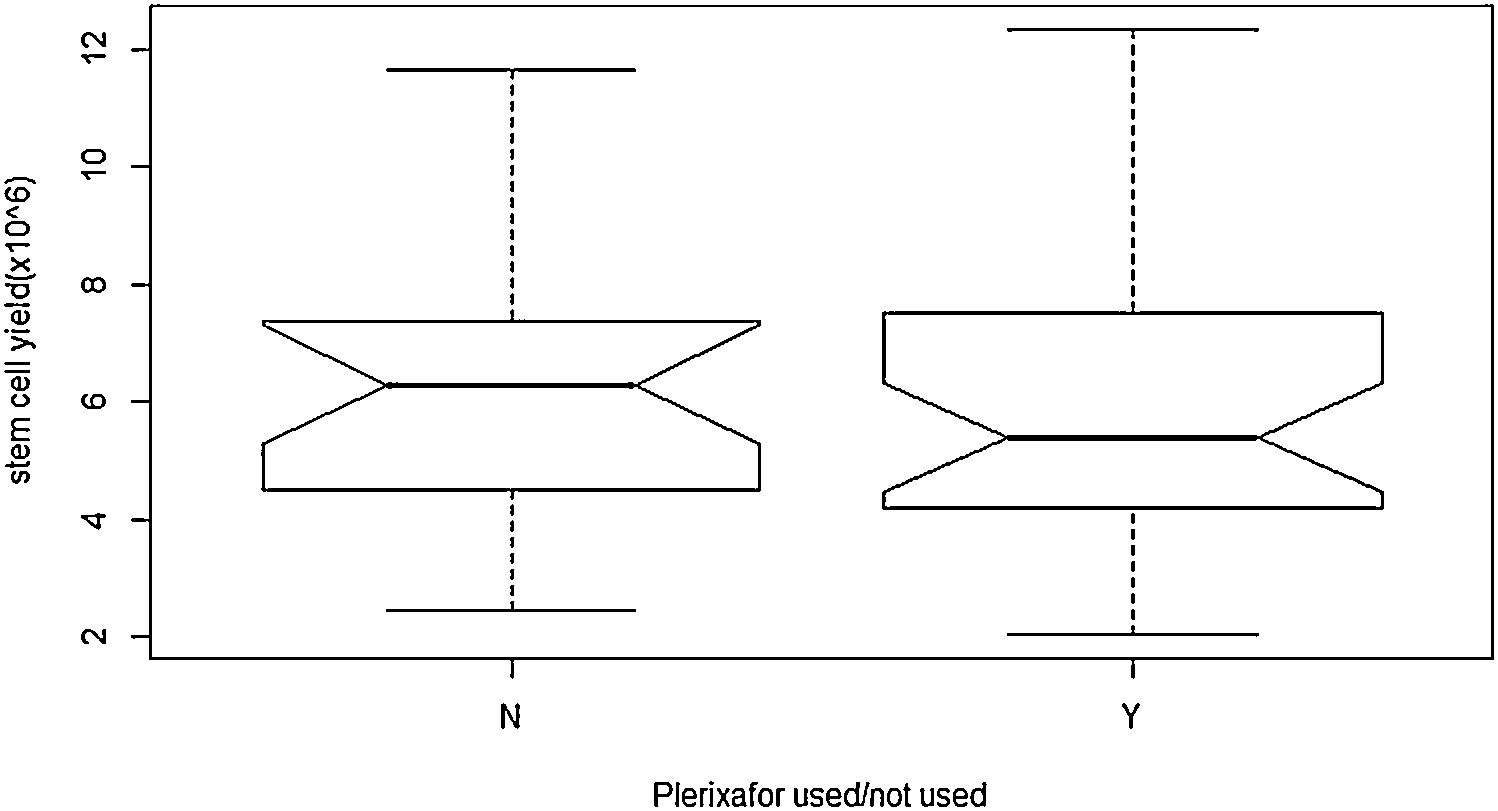
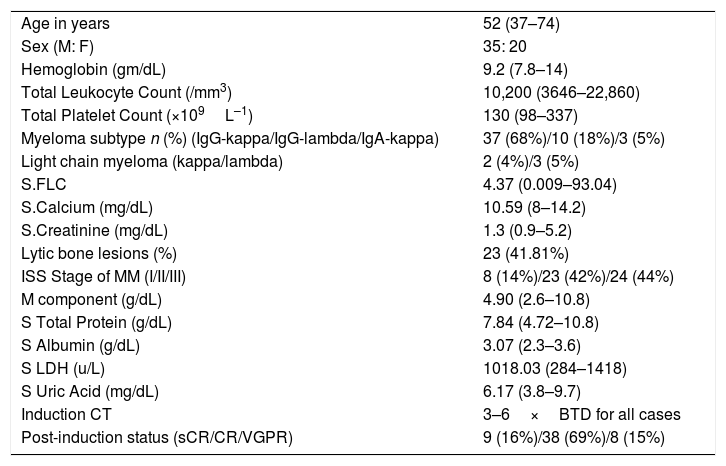
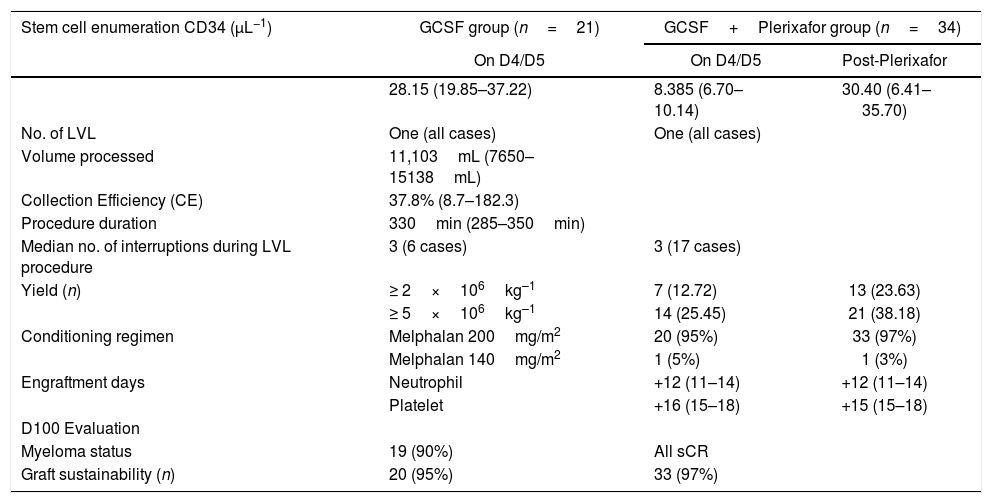
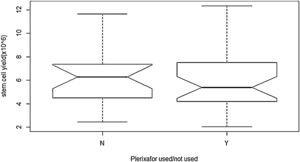
ObservationA total of 55 cases of NDMM (35 male and 20 female) were included in the present study. The baseline characteristics and post-induction myeloma status are presented in Table 2. Forty-seven cases (85%) achieved sCR/complete response (CR), while rest achieved VGPR after the induction phase of the treatment. Details of stem cell mobilization, conditioning regimen, engraftment and post-ASCT response (+100 days) are shown in Table 3. Successful stem cell mobilization (primary efficacy endpoint) was achieved in all cases by a single LVL procedure; 21 cases with G-CSF alone and 34 cases with G-CSF plus Plerixafor. Interruption of the procedure due to various reasons (non-cooperation, bathroom urgency, rigor, blockage of access/return line, etc.) was seen in 6 cases and 17 cases in the G-CSF and G-CSF plus Plerixafor groups, respectively. The ideal stem cell yield of ≥5×106kg–1 CD34+ was achieved in 66.7% of the cases in the G-CSF group, vis-a-vis in 61.7%, in the G-CSF plus Plerixafor group, albeit higher median pre-apheresis CD34+ count in the latter. Although there was a significant increment in the CD34 count after the utilization of Plerixafor, there was no significant difference in the SC yield between these two groups (p=0.3982). (Figure 1) Standard high-dose melphalan was used as the conditioning regimen in both groups. Neutrophil/platelet engraftment, D100 myeloma status and graft sustainability were the same in both groups, i.e., 12 days vs. 16/15 days, 90%/100% sCR and 95%/97%, respectively. (Table 3)
Demographic and baseline characteristics of the patients (n=55).
| Age in years | 52 (37–74) |
| Sex (M: F) | 35: 20 |
| Hemoglobin (gm/dL) | 9.2 (7.8–14) |
| Total Leukocyte Count (/mm3) | 10,200 (3646–22,860) |
| Total Platelet Count (×109L–1) | 130 (98–337) |
| Myeloma subtype n (%) (IgG-kappa/IgG-lambda/IgA-kappa) | 37 (68%)/10 (18%)/3 (5%) |
| Light chain myeloma (kappa/lambda) | 2 (4%)/3 (5%) |
| S.FLC | 4.37 (0.009–93.04) |
| S.Calcium (mg/dL) | 10.59 (8–14.2) |
| S.Creatinine (mg/dL) | 1.3 (0.9–5.2) |
| Lytic bone lesions (%) | 23 (41.81%) |
| ISS Stage of MM (I/II/III) | 8 (14%)/23 (42%)/24 (44%) |
| M component (g/dL) | 4.90 (2.6–10.8) |
| S Total Protein (g/dL) | 7.84 (4.72–10.8) |
| S Albumin (g/dL) | 3.07 (2.3–3.6) |
| S LDH (u/L) | 1018.03 (284–1418) |
| S Uric Acid (mg/dL) | 6.17 (3.8–9.7) |
| Induction CT | 3–6×BTD for all cases |
| Post-induction status (sCR/CR/VGPR) | 9 (16%)/38 (69%)/8 (15%) |
Stem cell mobilization, conditioning regimen, engraftment and post-ASCT response.
| Stem cell enumeration CD34 (μL−1) | GCSF group (n=21) | GCSF+Plerixafor group (n=34) | |
|---|---|---|---|
| On D4/D5 | On D4/D5 | Post-Plerixafor | |
| 28.15 (19.85–37.22) | 8.385 (6.70–10.14) | 30.40 (6.41–35.70) | |
| No. of LVL | One (all cases) | One (all cases) | |
| Volume processed | 11,103mL (7650–15138mL) | ||
| Collection Efficiency (CE) | 37.8% (8.7–182.3) | ||
| Procedure duration | 330min (285–350min) | ||
| Median no. of interruptions during LVL procedure | 3 (6 cases) | 3 (17 cases) | |
| Yield (n) | ≥ 2×106kg–1 | 7 (12.72) | 13 (23.63) |
| ≥ 5×106kg–1 | 14 (25.45) | 21 (38.18) | |
| Conditioning regimen | Melphalan 200mg/m2 | 20 (95%) | 33 (97%) |
| Melphalan 140mg/m2 | 1 (5%) | 1 (3%) | |
| Engraftment days | Neutrophil | +12 (11–14) | +12 (11–14) |
| Platelet | +16 (15–18) | +15 (15–18) | |
| D100 Evaluation | |||
| Myeloma status | 19 (90%) | All sCR | |
| Graft sustainability (n) | 20 (95%) | 33 (97%) | |
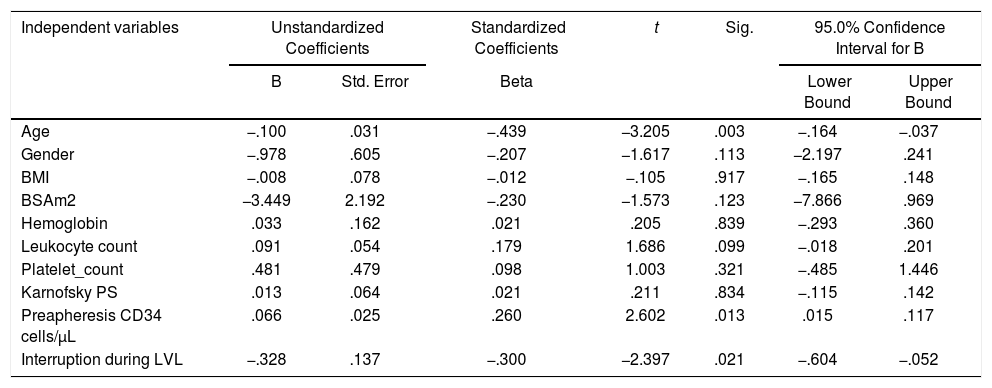
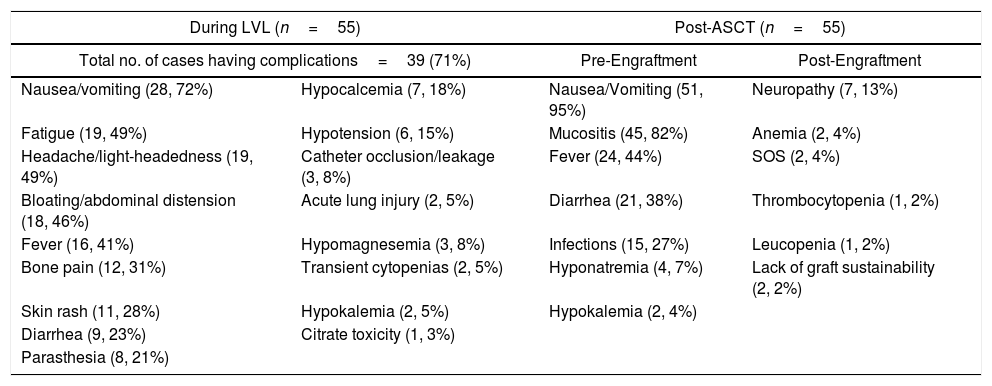
Table 4 depicts the linear regression model, in which the adjusted R2=0.560 showed that the 56% variance in the dependant variable, i.e., the stem cell yield, can be explained conjunctively by all the independent variables, such as age, gender, BMI, BSA, hemoglobin, leukocyte count, platelet count, Karnofsky score, pre-apheresis CD34 count and no interruption during apheresis. The number of interruptions during the procedure, pre-apheresis CD34 count and age of the patients were found to be significant factors (p<0.05), in increasing order, influencing the stem cell yield in the regression analysis (Table 4). The ADRs were reported in 39 cases (71%) during the LVL procedure (Table 5), which were of mild to moderate grade, and were managed successfully with standard procedures in all cases. Gastrointestinal disturbances (nausea and vomiting), mucositis, fever and infection were common complications encountered in the post-ASCT period (Table 5) and were successfully managed by following the standard treatment in all cases. We could not detect any difference in the incidence or severity of any ADRs during the LVL procedure or post-ASCT period between these two groups (G-CSF vs. G-CSF plus Plerixafor).
Factors affecting LVL.
| Independent variables | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| Age | −.100 | .031 | −.439 | −3.205 | .003 | −.164 | −.037 |
| Gender | −.978 | .605 | −.207 | −1.617 | .113 | −2.197 | .241 |
| BMI | −.008 | .078 | −.012 | −.105 | .917 | −.165 | .148 |
| BSAm2 | −3.449 | 2.192 | −.230 | −1.573 | .123 | −7.866 | .969 |
| Hemoglobin | .033 | .162 | .021 | .205 | .839 | −.293 | .360 |
| Leukocyte count | .091 | .054 | .179 | 1.686 | .099 | −.018 | .201 |
| Platelet_count | .481 | .479 | .098 | 1.003 | .321 | −.485 | 1.446 |
| Karnofsky PS | .013 | .064 | .021 | .211 | .834 | −.115 | .142 |
| Preapheresis CD34 cells/μL | .066 | .025 | .260 | 2.602 | .013 | .015 | .117 |
| Interruption during LVL | −.328 | .137 | −.300 | −2.397 | .021 | −.604 | −.052 |
Dependant variable: stem cell yield (106).
Adverse reactions and complications.
| During LVL (n=55) | Post-ASCT (n=55) | ||
|---|---|---|---|
| Total no. of cases having complications=39 (71%) | Pre-Engraftment | Post-Engraftment | |
| Nausea/vomiting (28, 72%) | Hypocalcemia (7, 18%) | Nausea/Vomiting (51, 95%) | Neuropathy (7, 13%) |
| Fatigue (19, 49%) | Hypotension (6, 15%) | Mucositis (45, 82%) | Anemia (2, 4%) |
| Headache/light-headedness (19, 49%) | Catheter occlusion/leakage (3, 8%) | Fever (24, 44%) | SOS (2, 4%) |
| Bloating/abdominal distension (18, 46%) | Acute lung injury (2, 5%) | Diarrhea (21, 38%) | Thrombocytopenia (1, 2%) |
| Fever (16, 41%) | Hypomagnesemia (3, 8%) | Infections (15, 27%) | Leucopenia (1, 2%) |
| Bone pain (12, 31%) | Transient cytopenias (2, 5%) | Hyponatremia (4, 7%) | Lack of graft sustainability (2, 2%) |
| Skin rash (11, 28%) | Hypokalemia (2, 5%) | Hypokalemia (2, 4%) | |
| Diarrhea (9, 23%) | Citrate toxicity (1, 3%) | ||
| Parasthesia (8, 21%) | |||
This was a single-center prospective study on a uniform cohort of newly diagnosed multiple myeloma cases, from whom we achieved a 100% success rate in harvesting the minimum target stem cell yield of 2×106 CD34+ cells/kg in a single setting of LVL from peripheral blood. The baseline characteristics and the post-induction response status were evenly matched between the G-CSF and G-CSF plus Plerixafor groups. Generic formulation of G-CSF and Plerixafor are safe, well tolerated, effective and have no adverse effect on engraftment, nor on graft sustainability at D100 of the post-transplant. In addition, we have also categorically demonstrated that LVL is safe, cost-effective and has a minimum adverse effect, which can be managed by following standard protocols. This is corroborated by the cost-effectiveness results of other studies.12,13 These findings have a significant importance in developing counties, in which the cost can be reduced to a great extent if the target cell yield is achieved in single apheresis procedure.
The success rate of the minimum yield of ≥2×106 CD34+ cells/kg by using G-CSF and G-CSF plus Plerixafor have been reported by DiPersio et al. and Pusic et al. as follows: 47.3% vs. 86.7% and 81.2% vs. 81.4%, respectively.17,18 The 100% success rate of achieving the primary endpoints in our study could be explained due to various factors, such as the following: all cases in the study were NDMM; efficient administration of induction CT with novel agents; achievement of deeper response (sCR/CR in 85% of the cases); pre-emptive uses of plerixafor when D4 CD34+ cell count was <10μL−1, and; judicious intervention of trained apheresis experts during the entire period of the apheresis procedure. The VTD regimen is one of the most efficient induction CTs, which has been considered as a standard protocol and was administered in all our cases. The median number of the induction CT was 5 (range 3–6), which correlates with a good depth of response in 85% of the cases (at the end of the induction CT). The intervention was made to match the color of the mononuclear cell collection (MNC) tube with the colorigram in all the cases of both groups. This fact also contributed to the high success rate of cell yield in this study.
The achievement of the high success rate of all the secondary efficacy endpoints is another important aspect of our study. The median neutrophil engraftment and platelet engraftment days were 12 and 16 and 12 and 15 in the G-CSF and G-CSF plus plerixafor groups, respectively, which are in accordance with other studies.17,19 The D100 graft sustainability was very high in both the groups (95% and 97%, respectively). The ASCT has improved the depth of response in both the groups by achieving sCR in 90% and 100% of the cases, respectively. We could not ascertain the exact depth of response, as the MRD (minimal residual disease) was not measured due to the lack of such facilities at our institution. The target of ≥5×106 CD34+ cells/kg was selected as one of the secondary endpoints to ensure the option of a possible tandem ASCT, in which two 2×106 CD34+ cells/kg transplants could be possible. This factor has a relevance to institutions (including ours), in which a liquid nitrogen storage facility is available so that high risk myeloma patients or relapsed patients (two years after the 1st transplant) may be benefited from a tandem ASCT or 2nd ASCT, respectively.20–22 This target was achieved in 66.7% of the cases in the G-CSF group and 61.7% of the G-CSF plus Plerixafor group, which were quite satisfactory, in comparison with another study by John F. DiPersio et al., who achieved it in 34.4% of the G-CSF group and 71.6% of G-CSF plus Plerixafor group, by employing 2 or less conventional HPC(A) procedures.17
Different covariates, such as age, gender, BMI, BSA, Hb, leukocyte count, platelet count, Karnofsky PS, per-apheresis CD34 count and interruption during the LVL procedure, were studied with the stem cell yield in a Cox-regression analysis model. The age and number of interruptions during apheresis are two significant factors (p=0.003 and p=0.021, respectively), which are inversely related to the stem cell yield, while the pre-apheresis CD34 count is another significant factor (p=0.013), which is directly proportionate to the stem cell yield. Other factors could not be considered to have a significant impact on the CD34+ yield. The pre-transplant depth of response has been correlated directly with the stem cell yield by other studies, but we failed to appreciate it. This could be due to the fact that the pre-transplant myeloma response was very similar in both groups, i.e., 100% sCR in the G-CSF + Plerixafor group and 90%, in G-CSF group. The age and pre-apheresis CD34 count have been significantly well correlated with the CD34 yield by various studies.23,24 Judicious intervention and counseling/motivation of the patients to minimize the interruption during apheresis could be an important factor in achieving stem cell yield, a new finding revealed by the present study. This finding could be explained by the fact that the interface created by the apheresis machine is disturbed when the procedure is halted and the re-establishment of the interface takes time and the collection of stem cells is put on hold until the color of the MNC collecting tubing matches with the colorigram.
The generic molecules of both drugs used in the stem cell mobilization, i.e., G-CSF and Plerixafor are safe, well tolerated and effective, with very good engraftment and D100 depth of response/graft sustainability, which are comparable with other studies using the original molecules. The median duration of the LVL procedure was 330minutes. Gastrointestinal disturbances, such as nausea/vomiting (28.72%), bloating and abdominal distension (18.46%) and fatigue (19.49%), headache/light-headedness (19.49%), transient fever (16.41%) and bone pain (12. 31%) were common ADRs reported during the LVL procedure, while other events were reported in 3% to 18% of the cases (Table 5). All these adverse events were of mild to moderate severity and were managed by following the standard procedures. This suggests the safety of the LVL procedure, albeit with a long duration, vis-a-vis conventional HPC(A). Cytopenia (leukopenia and thrombocytopenia), gastrointestinal disturbances, mucositis, fever and infection were common ADRs during the post-ASCT period, which were consistent with the expected lines and in agreement with other studies (Table 5). All these events were managed by following standard care. The incidence, severity and successful management of ADRs were very similar in both groups. Two of our cases (out of the 34 in the G-SCF+Plerixafor group) developed myelodysplasia after D100 of the post-ASCT period, which explains the lack of graft sustainability. Its significance need be reascertained in larger groups in future studies.
ConclusionThe LVL is safe, effective and can achieve the required minimum of CD34+cells in a single apheresis, with minor adverse reactions, which can be easily managed by following the standard protocol. The number of interruptions during apheresis, pre-apheresis CD34 count and age are significant factors influencing the cell yield. Judicious intervention during the procedure, ensuring the quality of the CD34+ cell yield, by matching the colorigram may help in maintaining the adequate cell yield. All these factors are relevant to resource-constrained settings.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sector.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the apheresis technicians Mr. RN Sahoo, Mr. Debi Prasad Jena and Mr. Rashmi Ranjan Bhoi and the nursing staffs of the bone marrow transplant unit, namely Mrs. Snehalata Mangaraj, Mrs. Annapurna Samal, Mrs. Ranjeeta Jena and Mrs. Mandakini Behera.