Myeloid/lymphoid neoplasms (MLNs) with fibroblast growth factor receptor-1 (FGFR1) rearrangement are immensely rare hematological malignancies, which involve the chromosome 8 rearrangement.1-4 It is not unusual for these hematological malignancies to be misdiagnosed, with the most common initial diagnosis as myeloproliferative neoplasm (MPN), associated with rapid transformation within a few months after diagnosis to lymphoblastic lymphoma (LBL), or acute lymphoblastic leukemia (ALL), or acute myeloid leukemia (AML). With the rapid progression, the prognosis is very poor, despite intensive chemotherapy.1,2
Since the disease was described in 1995 by McDonald et al.,3 less than 100 cases have been reported worldwide.5 Here we describe the first case of the disease in Vietnam, a female patient with myeloid/lymphoid neoplasms associated with FGFR1 rearrangement, who had rapid progression of the disease. She experienced hyperleukocytosis, disseminated intravascular coagulation (DIC), pneumonia, and subdural hemorrhage. She eventually succumbed to the disease.
Case presentationA 20-year-old woman with a high fever and swollen gums for a week was admitted to the Blood Transfusion Hematology Hospital in Ho Chi Minh City, Vietnam. Nine months before the admission, she initially experienced lymphadenopathy and persistent fever. She was diagnosed with non-Hodgkin lymphoma (T-cell lymphoblastic lymphoma) stage IV and underwent chemotherapy with the Eastern Cooperative Oncology Group (ECOG) E2993 regimen with daunorubicin, vincristine and methylprednisolone for 3 months at another hospital. However, her condition was worsening, with no response to chemotherapy, so she postponed the chemotherapy. She and her family had no other medical problems.
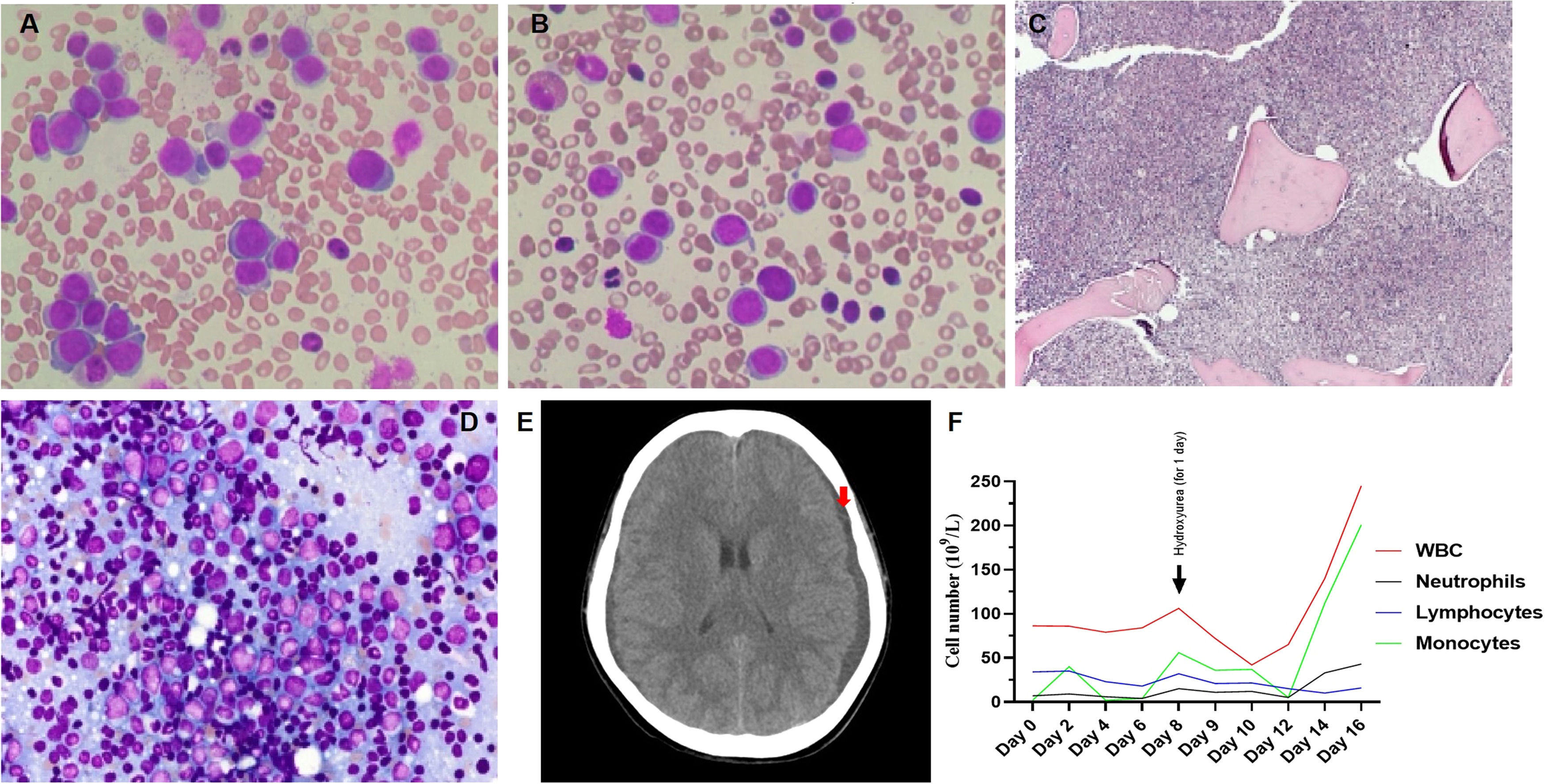
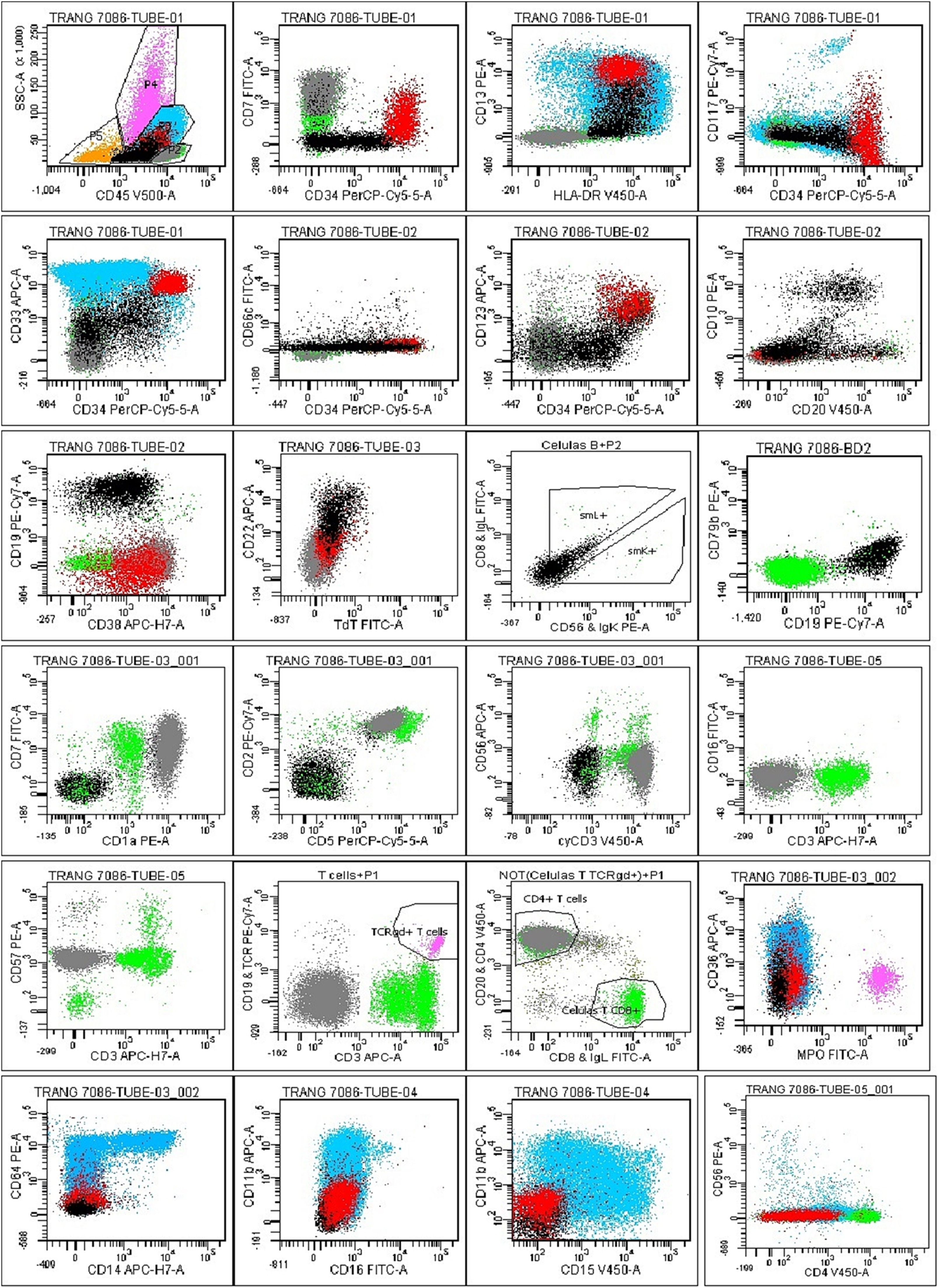
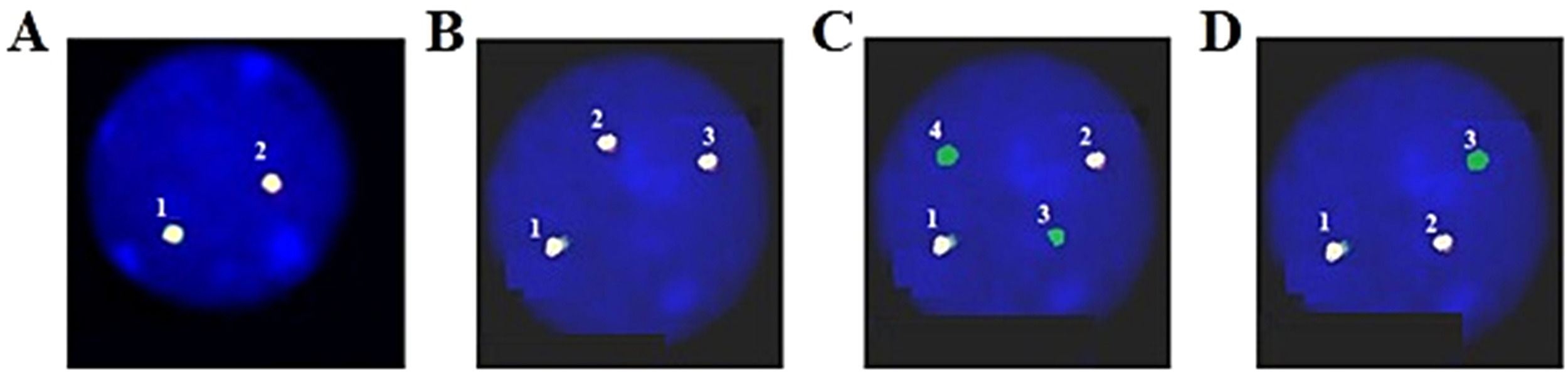
Upon examination, the blood pressure, pulse, temperature and respiration were 130/70mmHg, 80/min., 38.5°C and 20/min., respectively. She appeared chronically ill and had pale conjunctiva and swollen gum. There was palpable hepatosplenomegaly and lymphadenopathy. The complete blood count showed a white blood cell (WBC) count of 86.4 × 109/L (neutrophils: 9.1%, lymphocytes: 39.4%, monocytes: 1.4%, eosinophils: 2.4% and basophils: 2.8%), with platelets at 27 × 109/L and Hgb at 7.1 g/dL, with MCV: 76.6 fL and MCH: 22.5 pg. The peripheral blood smear showed a low amount of red blood cells and platelets, with a high amount of white blood cells (segment neutrophils: 15%, lymphocytes 35%, monocytes: 17%, segment eosinophils: 3%, and blasts: 30%) (Figure 1A). The blood chemistry showed the normal range of AST, ALT, Creatinin and total/direct bilirubin, but lactate dehydrogenase (LDH): 1,282 U/L and uric acid: 431umol/L. The coagulation profile revealed a prothrombin time of 19.1s. (control: 13.4s.), a partial thromboplastin time of 34.7s. (control: 29.5s.) and a fibrinogen level of 2.6 g/L. The hepatitis B and C, human immunodeficiency virus, Epstein-Barr virus and human T-lymphotropic virus type I và II antigens and antibodies were all negative. The bone marrow examination showed a hypercellular marrow, with 67% of blasts (Figures 1B and 1C) and an increased B cell population (Figure 1D). The concurrent flow cytometry analysis showed four distinguished populations, including B-lymphoblasts: 9% (CD45inter CD34+ CD10± TdT− CD19+ CD20± CD66c− CD123− CD13± CD38±), Myeoblasts: 7% (CD45inter CD34+ CD117± HLA-DR+ CD13+ CD33+ CD7± MPO− CD15− CD38±), T lymphocytes: 13% (CD45inter-bright CD34− CD10− CD7+ CD1a+ CD3– CD2+ CD5+ CD4+ CD8– CD56− CD57−), Monocytes: 52% (CD45bright CD34− CD117− MPO− HLA.DR+ CD64± CD14± CD15− CD36± CD4±) (Figure 2). An FGFR1 (8p11) rearrangement was detected using fluorescence in situ hybridization (FISH) with Cytocell FGFR1 Break apart/Amplification probe. Analyzing 200 cells from the peripheral blood showed that 20% of the cells are normal, 15% of the cells have 3 signals of FGFR1 (8p11), 25% of the cells have 2 signals of FGFR1 with 2 signals lost 3’ end of the FGFR1 and 40% of the cells have 2 signals of the FGFR1 with 1 signal lost 3’ end of the FGFR1 (Figures 3A, 3B, 3C and 3D). We did not have the opportunity to examine the translocation partner gene. For other molecular studies, reverse transcription polymerase chain reaction (RT-PCR) results showed no PML-PARA fusion. The karyotype results showed 47, XY, +14.
A. Peripheral blood smear showed a low amount of red blood cells and platelets, with a high amount of white blood cells, with 30% blasts. B. The bone marrow aspiration showed a hypercellular marrow, with 67% blasts. C. The bone marrow biopsy also showed a hypercellular marrow (H&E stain, original magnification × 100). D. Immunohistochemistry staining showed CD34 (+) 7%, CD3 (+) 10%, TdT (+) 20%, CD20 (+) < TdT(+), CD117 (+) 20%, PAX5 (+) > TdT (+), CD1a (+) 5%, CD10 (+) 10%, MPO (+) 15% and CD33 (+). E. The head CT scan showed new subdural hemorrhages on both temporal lobes (red arrow). F. The patient leukocytes graph from admission to discharge.
The flow cytometry analysis on bone marrow showed distinguished populations, including B-lymphoblast: 9% (black population) (CD45inter CD34+ CD10± TdT− CD19+ CD20± CD66c− CD123− CD13± CD38±), Myeoblasts: 7% (red population) (CD45inter CD34+ CD117± HLA-DR+ CD13+ CD33+ CD7± MPO− CD15− CD38±), T lymphocytes: 13% (grey population) (CD45inter-bright CD34− CD10− CD7+ CD1a+ CD3– CD2+ CD5+ CD4+ CD8– CD56− CD57−), Monocytes: 52% (blue population) (CD45bright CD34− CD117− MPO− HLA.DR+ CD64± CD14± CD15− CD36± CD4±). Mature myeloid cells 9.5% (pink population). Red blood cells and debris 4.5% (yellow population).
An FGFR1 (8p11) rearrangement was detected using fluorescence in situ hybridization (FISH) with Cytocell FGFR1 Break apart/Amplification probe. Analyzing 200 cells from peripheral blood showed that: A. 20% of cells are normal; B. 15% of cells have 3 signals of FGFR1; C. 25% of cells have 2 signals of FGFR1 with 2 signals lost 3’ end of FGFR1, and; D. 40% of cells have 2 signals of FGFR1 with 1 signal lost 3’ end of FGFR1.
She received blood transfusions and was treated with intravenous (IV) ceftazidime 100mg/kg/day and IV amikacin 15mg/kg/day for 8 days, but there were no improvements in the fever and swollen gums. On day 8 after hospital admission, her WBC count reached 106.5 × 109/L and she was treated with oral hydroxyurea 1g x 2 for one day. On day 9, she had a high fever, dyspnea, headache and drowsy mental status. She experienced disseminated intravascular coagulation (DIC), with a prothrombin time of 23s. (control: 13.4s.), a partial thromboplastin time of 38.7s. (control: 29.5s.), a fibrinogen level of 0.6 g/L and a D-Dimer of 13.2 µg/mL, as well as developing new subdural hemorrhages on both temporal lobes in the CT scan (Figure 1E) and pneumonia. She was immediately transferred to the intensive care unit, placed on supported breathing by nasal continuous positive airway pressure (NCPAP) and treated with IV meropenem 1g x 3/day, IV amikacin 15mg/kg/day and IV amphotericin B deoxycholate 1mg/kg/day. Unfortunately, her condition continually worsened, with her WBC count reaching 245 × 109/L (neutrophils: 13.9%, lymphocytes: 4.4% and monocytes: 81.6%) on day 16 (Figure 1F) and she was discharged, following her family's wishes, on day 17.
DiscussionThe aim of this study was to raise awareness and share our experience in the MLN with the FGFR1 rearrangement. Here, we report the first case of the disease in Vietnam. It is not unusual for this hematological malignancy to be misdiagnosed and have a rapid progression and very poor prognosis. Furthermore, this hematological malignancy has no established standard therapy, with allogeneic hematopoietic stem cell transplantation (aSCT) being the only curative treatment option.1,2
As there is no established standard therapy for the MLN with the FGFR1 rearrangement and the previous reports on the disease used various treatments, including the ALL and AML regimens,1,2 but, despite the high-intensity chemotherapy, the response rate was very low, suggesting that the FGFR1 rearrangement may be associated with chemoresistance.2,6 Recently, with the introduction of the targeted therapy, such as the FGFR kinase inhibitor Pemigatinib, which may offer a functional cure for the MLN with the FGFR1 rearrangement,1 as the use of the tyrosine kinase inhibitor Imatinib does in the case of chronic myeloid leukemia. The FGFR kinase inhibitor can also serve as a pathway to the aSCT, or as an option for patients not eligible for the aSCT. An investigation of FGFR kinase inhibitors in patients with the MLN with the FGFR1 rearrangement is still ongoing (ClinicalTrials.gov: NCT03011372). The newest analysis of the ongoing clinical trials (NCT03011372) showed that pemigatinib is safe and can induce high and durable rates of complete responses and complete cytogenetic responses in patients with the MLN.7 Ponatinib, a multi-targeted tyrosine-kinase inhibitor, with immense promising pre-clinical data in the myeloid neoplasm with FGFR1-rearranged tumors,8,9 has also been used in combination with chemotherapy for the treatment of one patient with FGFR1-rearranged acute leukemia and achieved a complete response.6 Furthermore, in one of the largest reports regarding the MLN with the FGFR1 rearrangement, Paolo Strati et al. reported that 78% of these patients had a coexisting mutation of the RUNX1, a gene well recognized for a poor prognosis in acute leukemia (1). This result suggests that the RUNX1 may be an option for the novel targeted therapy.1 These reports suggested that the combination between targeted therapy and chemotherapy is more effective to treat the MLN with the FGFR1 rearrangement.1,2 These treatment options can serve as a bridge from the initial diagnosis to disease stabilization and to the aSCT.
In summary, we would like to share our experience with the MLN with the FGFR1 rearrangement, which is a very rare entity and should be considered when the clinical phenotype is heterogeneous, involving myeloid and lymphoid lineages or with extramedullary involvement.10 Its correct diagnosis and induction therapy is crucial. Chemotherapy in combination with targeted therapy may further improve the response of the patients and serve as a bridge to the aSCT.
Ethical approvalThis study was approved by our hospital Institutional Review Board.
Informed consentThe written consent form was acquired from the patient prior to the study.
Consent for publicationThe written consent form was acquired from the patient prior to the study.