The development of red blood cells (RBCs), or erythropoiesis, occurs in specialized niches in the bone marrow, called erythroblastic islands, composed of a central macrophage surrounded by erythroblasts at different stages of differentiation. Upon anemia or hypoxemia, erythropoiesis extends to extramedullary sites, mainly spleen and liver, a process known as stress erythropoiesis, leading to the expansion of erythroid progenitors, iron recruitment and increased production of reticulocytes and mature RBCs. Macrophages are key cells in both homeostatic and stress erythropoiesis, providing conditions for erythroid cells to survive, proliferate and differentiate. During RBCs aging and injury, macrophages play a fundamental role again, performing the clearance of these cells and recycling iron for new erythroblasts in development. Thus, macrophages are crucial components of the RBCs turnover and in this review, we aimed to cover the main known mechanisms involved in the process of birth and death of RBCs, highlighting the importance of macrophage functions in the whole RBC lifecycle.
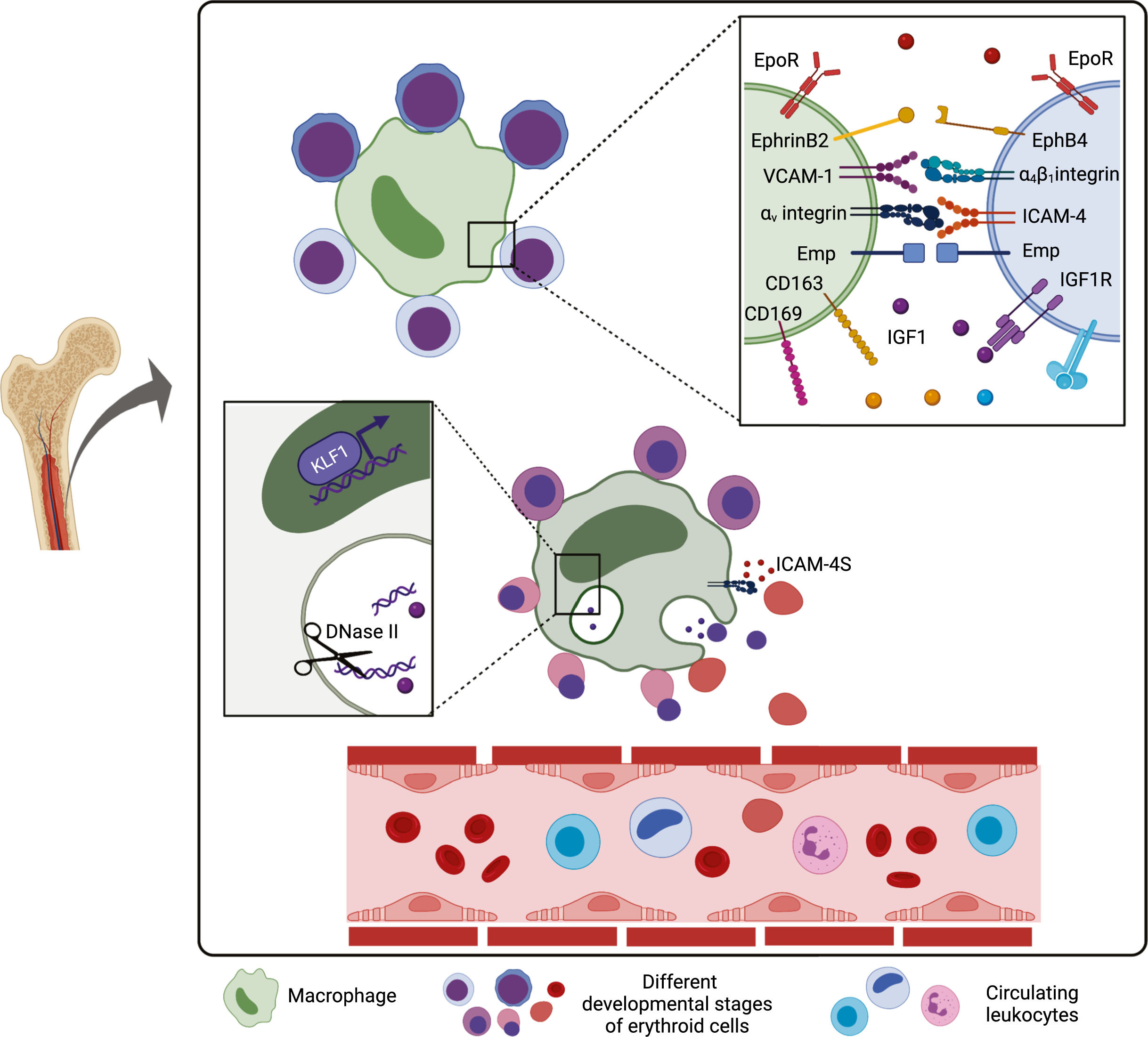
Erythropoiesis is a dynamic and complex process responsible for the production of approximately 200 billion red blood cells (RBCs) daily. It occurs in specialized niches in the bone marrow called erythroblastic islands (EBIs), which are composed of a central macrophage surrounded by erythroblasts at different stages of differentiation.1,2 Throughout the erythropoiesis in this microenvironment, there is a progressive drop in the proliferative capacity of progenitor cells, which differentiate into proerythroblasts, undergo cytoplasmic maturation and nuclear changes that generate, in sequence, basophilic, polychromatophilic and orthochromatic erythroblasts. The latter expels their nuclei, becoming reticulocytes, and finally mature erythrocytes or RBCs, which circulate in the bloodstream until their senescence (Figure 1).
Erythropoiesis process. Each proerythroblast is capable of generating 16 erythrocytes upon erythropoiesis. The proliferative rate drops throughout the erythroid cell development, as well as the cell size, whereas the chromatin undergoes progressive condensation. There is an mRNA accumulation in the basophilic erythrocytes, which gives them the blue color upon staining. As cell maturation progresses, hemoglobin synthesis is increased, giving the pinkish/red color conferred by iron molecules, which mixes with blue color of mRNA in intermediate phases (polychromatic erythroblasts). The nucleus is extruded in the orthochromatic phase, generating the reticulocytes and further maturation, with the loss of the rest of the RNA and organelle content, giving rise to mature erythrocytes. Created with BioRender.com.
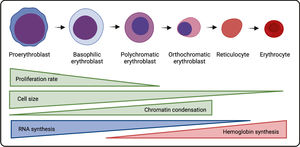
The EBI environment is fundamental for this sequence of events, as the level of erythropoietic activity is directly associated with the number of islands. Macrophages are the centerpieces of this process, with essential physiological functions, including the supply of iron for hemoglobin synthesis, phagocytosis of the expelled nuclei during erythroblast enucleation, and induction of erythroblast proliferation and survival.3,4 At least some of these effects seem to be mediated in a manner dependent on contact of the macrophage with the erythroblast,5 underlining the importance of adhesion molecules and paracrine factors secreted within the erythroid niches for this regulatory event.
Macrophages are heterogeneous cell types with phenotypes and functions that vary within a spectrum, from classically activated M1 and alternatively activated M2 macrophages. The macrophage polarization is influenced by the molecules by which they are activated and the cytokines in the microenvironment. The lipopolysaccharide and the IFN-γ, for instance, drive the polarization of M1 macrophages, which induces a pro-inflammatory response with secretion of the tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and IL-1β necessary to kill some pathogens, whereas M2 macrophages are polarized by the IL-4 and IL-13 and are anti-inflammatory, producing the IL-10 and TGF-β, which are important for repairing the tissue from inflammation-induced injury. Moreover, macrophages can play distinct roles and present changes in the phenotype dependent on the tissue in which they reside.6 Evidence has suggested that EBI macrophages are also not a homogeneous population or, at least, are not in the same stage of maturation, and that their functions are related to the specific localization in the bone marrow. Macrophages located throughout the bone marrow are more likely to be attached to proerythroblasts and erythroblasts in the early stages of differentiation, whereas macrophages closed to sinusoids are intimately related to erythroblasts in the late stages.7 It is still uncertain whether these macrophages are distinct cells or if they migrate towards sinusoids and maturate as the erythropoiesis advances.
Macrophages in EBI structures have the M2-like phenotype with an anti-inflammatory profile 8 and they express the CD1693 and interact with erythroblasts by binding the α4β1 integrin, ICAM-4 and Emp (erythroblast macrophage protein) on erythroblasts with the VCAM-1 (CD106), αV integrin and Emp on macrophages, respectively.9 The role of these set of adhesion molecules in contributing to island integrity was demonstrated by the findings of severe anemia in Emp knockout (KO) mice fetuses,10 as well as the reduced number of islands in ICAM-4 KO mice 11 or when mice were treated with the anti-α4β1 integrin antibody.12 It is noteworthy that a secreted form of the ICAM-4, ICAM-4S is up-regulated in erythroblasts at the late stages of differentiation and it is postulated that it competes with the membrane ICAM-4 by binding with the αV integrin in macrophages. Thus, immature reticulocytes can disconnect from macrophages and enter the bloodstream.13 It was shown that the CD169, VCAM-1 and F4/80, well-recognized markers of the macrophage, are heterogeneously expressed by EBI macrophages, which might represent phenotyping differences of EBIs located in distinct zones of the bone marrow.14
Recently, it has been proposed that the Ephrin/Eph receptor interaction in the EBI niche is important for the erythroblast differentiation. The inhibition of the interaction between ephrin-B2, expressed by macrophages, and EphB4, which is expressed by erythroblasts and reticulocytes, resulted in a decrease in the association between these two cell types, demonstrating the importance of this interaction for the stability of the EBIs.15 Human EBI macrophages also express the CD163, a receptor for the hemoglobin-haptoglobin complex, which, besides being responsible for clearing the cell-free hemoglobin, can act as an adhesion molecule to erythroblasts, although its ligand is still unknown.16 Interesting, it has been shown that, like erythroid cells, 90% of EBI macrophages express the erythropoietin receptor, EpoR, both in mice and in the human fetal liver and thus, the Epo can act simultaneously in both cell types, improving the EBI formation, at least in part, by increasing the expression of adhesion molecules in macrophages.17
Soluble factors, secreted by macrophages, also contribute with specific stages of erythropoiesis in the EBI microenvironment, as the culture of erythroblasts have higher proliferative activity when macrophages are present, in part due to supernatant molecules. Although all the factors produced by macrophages in the EBI niche are not completely known, it was shown that the proliferation of erythroid cells induced by macrophages is independent of the Epo,5 the main mediator involved in the RBC production. Recent efforts aiming to reveal these factors showed that EBI macrophages express the insulin-like growth factor (Igf1) and Il-18,17 suggesting a role for these mediators in either proliferation, survival or differentiation of erythroid cells. In fact, erythroblasts in several development stages express the IGF1 receptor (IGF1R) and the IGF1 has already been shown to increase erythropoiesis.18 Furthermore, the expression of the IL-18R was also seen in erythroid linage cells,19 although the role of the IL-18 secreted in the EBI has not been investigated. Hence, although novel important studies are revealing previously unknown soluble factors involved in the RBC development, more studies are still needed to uncover other possible mediators and growth factors produced by the macrophage, their cognate receptors in erythroblasts and which stages of the erythropoiesis process they may influence.
Some intracellular proteins of EBI macrophages were identified as important players in erythropoiesis. With erythroblasts attached to macrophages by adhesion molecules, the latter can support the erythrocyte differentiation by providing the iron required for hemoglobin synthesis. Several proteins are involved in the iron recycling and exporting by macrophages and they will be discussed in the last section of this review. Additionally, during maturation of erythroblasts into reticulocytes, a great number of nuclei and organelles are excluded by these cells. The extruded nuclei, known as pyrenocytes, are phagocyted by EBI macrophages, which recognize phosphatidylserine (PS) by mechanisms similar to apoptotic cell recognition.4 In fact, the MFG-E8 (that binds to PS) mutant mice have impaired phagocytosis of both apoptotic cells and pyrenocytes from erythroid cells.20 Subsequently, after being phagocyted, nuclear and mitochondrial DNA are digested by the DNase II, expressed by central macrophages, an enzyme that is essential for life, as in the absence of the DNase II in the KO mice model all phagocyted DNA is not degraded and becomes accumulated within macrophages, resulting in the death of these cells and, consequently, inhibited erythropoiesis and lethal anemia.21
Macrophages are also responsible for the mitochondria clearance from erythroblasts during their maturation. The mechanism of the mitochondria transfer was demonstrated to be dependent on tunneling nanotubes and the depletion of murine EBIs macrophages resulted in the accumulation of mitochondria in erythroid cells and the reduction of mature RBCs.22 Interestingly, monocyte-derived macrophages were reported to influence the balance between the gamma and beta globin expression in the early stages of erythropoiesis in a contact-dependent manner. The presence of monocytes/macrophages in the erythroid culture gives rise to cells with lower fetal hemoglobin (HbF) than in the absence of them, although more studies are still necessary to clarify the mechanisms involved in the process.23
The expression of all proteins in a specific cell is orchestrated by a set of transcription factors that bind to the target genes, inducing or inhibiting their transcription. The transcription factor that best characterizes EBI macrophages is the KLF1 (Kruppel-Like Factor 1).17 The deficiency of the KLF1 is not compatible with life, as the KLF1 KO mice embryos die of severe anemia. The KFL1 was found to bind directly to the DNase II promoter and the KLF1 KO embryos have an extremely reduced DNase II expression,24 which may explain their phenotype. In addition, studies conducted using the KLF1-GPF mice or inducing the KFL1 in human macrophages in vitro revealed that the KFL1 is responsible for several features of EBI macrophages, such as expression of the VCAM-1, CD163 and CD169, in addition to the DNase II, and enables macrophage to induce the erythroid maturation.25 Other transcription factors have been identified by the transcriptome in EBI macrophages17 and future studies may clarify whether they play a role in the erythroid cell development. Figure 2 illustrates the EBI structure in the bone marrow and some of the membrane, secreted and intracellular molecules involved in erythropoiesis.
Erythroblast island niche in bone marrow. Macrophages are bound to erythroid cells in different stages of differentiation in the bone marrow. Transmembrane, soluble and intracellular molecules involved in erythropoiesis mediated by macrophages are depicted in this figure and their functions are detailed in the text. Created with BioRender.com.
Although erythropoiesis is an extremely active process during homeostatic situations, it can be further stimulated under conditions of anemia or hypoxemia, a process known as erythropoiesis in stress. During stress erythropoiesis, erythroid development extends to extramedullary sites, mainly the spleen and liver, leading to the expansion of erythroid progenitors, iron recruitment and increased production of reticulocytes and mature erythrocytes.26 Infection and inflammation play an important role in the development of anemia through the stimulation of toll-like receptors signaling and pro-inflammatory cytokine secretion, which drives hematopoiesis towards myeloid cell development in detriment of erythroid cells. Additionally, pro-inflammatory cytokines can increase both erythrophagocytosis and the proliferation of the extramedullary erythroblasts.27
Another mechanism stimulated by anemia is the production of Epo by the kidney cells. Epo acts both in erythroid progenitors and macrophages through EpoR, increasing erythropoiesis, although the signaling pathways activated in these cells seem to be different from each other. Accordingly, Epo induces the upregulation of anti-apoptotic genes in erythroid cells, providing their survival, whereas new EBI niches arise in the spleen by increasing the number of EpoR+ macrophages and their expression of adhesion molecules via the production of the prostaglandin E2.28 The stimulation of EpoR in macrophages is critical for erythropoiesis in non-physiological situations, as depletion of Epo/STAT5 signaling specifically in macrophages impairs stress erythropoiesis. Additionally, in stress situations with low iron stocks, red pulp macrophages (RPM) secrete the chemokine CCL2, which attract circulating monocytes to the spleen, where they differentiate in EBI macrophages. These new EBI structures formed in the spleen support the extension of erythropoiesis, restoring the levels of iron, in a CCR2-dependent manner, since the CCR2 KO mice showed a decline in both monocyte recruitment and erythroid cell expansion.29 A similar mechanism seems to occur in the liver, where Kupffer cells were shown to express EpoR and to produce CCL2 upon Epo stimulation, recruiting CCR2+ monocytes and supporting the extramedullary erythropoiesis.30
It has been demonstrated that the differentiation of erythrocytes in vitro from human CD34+ cells is 4 to 15 times greater when in the presence of other peripheral blood mononuclear cells (PBMCs). The cells responsible for this increase in erythroid differentiation were found to be monocytes, mainly the intermediate CD14highCD16+ monocytes, which express the mannose receptor (CD206), expressed by the anti-inflammatory M2-like macrophages, CD163 and CD169, resembling features of EBI macrophages. These data indicate that circulating monocytes may play some functions of EBI macrophages, especially in situations when intermediate monocytes are increased. The effect on erythroid differentiation promoted by monocytes is independent on contact and occurs before the erythroblast stage, increasing the proliferation and decreasing apoptosis of CD34+ cells.31 The factor present in the culture responsible for the differentiation of monocytes into macrophages with the phenotypic profile of EBI macrophages was found to be glucocorticoid. In vitro differentiation of macrophages from monocytes in the presence of dexamethasone was able to generate cells that interact with erythrocytes, promote erythropoiesis and phagocyte the nuclei expelled during the differentiation.8
The pathophysiology of some hemolytic anemias resembles that of a chronic state of stress erythropoiesis. These include two apparently dichotomous diseases, polycythemia vera and β-thalassemia. Polycythemia vera is characterized by extremely elevated erythrocytosis, associated with a constitutively active mutation in the JAK2 gene, which encodes a protein that plays a role in the EpoR signaling.32 In contrast, β-thalassemia is characterized by anemia due to the low β-globin production and expansion of erythroid progenitors, which is attributed to hundreds of mutations in the β-globin gene and its promoter.9 Both diseases share characteristics of erythropoiesis under stress, such as splenomegaly, expansion of erythroid progenitors and elevated reticulocytosis. An incisive contribution of macrophages in the pathology of polycythemia vera and β-thalassemia was shown in mice models, as macrophage depletion was able to reverse some key characteristics linked to the two diseases.33 The macrophages showed to be essential for the development of extramedullary erythropoiesis and splenomegaly presented by mice with these disorders. Mouse erythrocytes also had a longer lifespan in the absence of macrophages. Although the role of CD169 in homeostatic erythropoiesis is still not completely clear, the depletion of CD169+ macrophages in the polycythemia vera mice decreased the RBC development,3 showing the importance of these cells in stress erythropoiesis and reveling them as targets for future therapies.
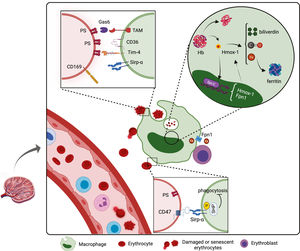
Clearance of senescent or damaged RBCs is performed by macrophagesOnce RBCs are released in the bloodstream, they perform their function of supplying O2 to body tissues and releasing the CO2 to the lungs for approximately 120 days. Senescent or damaged RBCs are then cleared mainly by the RPMs from the spleen in a constitutive manner.34 Macrophages can recycle the iron from the hemoglobin molecules of these cells, providing iron for new developing erythroblasts and therefore, the iron cycle restarts with macrophages playing an essential function in this process from the beginning to the end of erythrocyte lifespan.
Macrophages can recognize senescent or damaged RBCs by their absence of “self” markers or by the presence of “eat me” signs. All healthy cells express the molecule CD47, which binds to the Sirp-α (CD172a), expressed mainly by RPMs. The Sirp-α has ITIM motifs in the cytoplasmic tail that are phosphorylated after binding to the CD47 and induces an inhibitory intracellular signaling that impairs phagocytosis of CD47-expressing cells. This inhibitory signaling requires the recruitment and activation of the phosphatases SHP-1 and SHP-2. Interestingly, senescent RBCs progressively lose the CD47 expression and become target for clearance by Sirp-α+ macrophages in the spleen.35 As evidence of this, clearance of transfused CD47-deficient RBCs is extremely higher, compared with the clearance of transfused CD47+ RBCs. Intriguingly, the parasite plasmodium was seen to infect more efficiently young RBCs, which express higher levels of CD47, delaying their clearance by macrophages.36 Furthermore, the Sirp-α mutant mice had severe anemia and splenomegaly due to the high clearance of RBCs and stress erythropoiesis in the spleen,37 hence the CD47-Sirp-α binding is an exceptional target for the therapeutic approach.
In addition, damaged or senescent RBCs expose the PS at the outer cellular membrane, which is recognized by a set of receptors expressed mainly by RPMs and liver macrophages, such as TIM4, CD36 and TAM receptors. The latter comprises receptors, such as the Axl and MertK, that bind to the PS indirectly through the soluble molecule Gas6.38,39 Accordingly, RBCs from patients with SCD and thalassemia, which have a strikingly shortened lifespan, express higher levels of PS in the outer membrane than heathy individuals.40,41
Another characteristic acquired upon aging or injury by RBCs is the loss of elasticity and higher rigidity promoted by the oxidative stress and dehydration throughout their lives. These changes also occur and are particularly important in some genetic disorders, such as sickle cell disease (SCD), spherocytosis and thalassemia.42 Although it is not understood how the RBC rigidity can be recognized by macrophages, it was shown to prevail even over the recognition of the CD47.43 Recent findings shed light on how the erythrophagocytosis process takes place by showing that aged RBCs express adhesion molecules that allow them to bind to the extracellular matrix in the spleen architecture, promoting their retention in the organ, which results in RBC shrinkage and hemolysis. The resulting erythrocyte ghosts, instead of intact aged RBCs, are the ones able to be recognized and phagocyted by RPMs.44
After their recognition, RBCs are internalized and digested by macrophages. The digestion of phagocyted RBCs, as well as the endocyted complex hemoglobin-haptoglobin or heme-hemopexin by EBI macrophages and RPMs, generates the heme that is transported to the cytosol by the heme transporter HRG1, which in turn is post-transcriptionally regulated by the presence of heme and iron.45 The endogenous labile heme binds to and releases the transcription factor SPI-C from its repressor BACH1. Free SPI-C migrates to the nucleus where it up-regulates the expression of important genes that code proteins involved in the iron metabolism, such as the enzyme heme oxygenase-1 (Hmox-1) and the iron exporter ferroportin 1 (Fpn1).46 Therefore, SPI-C enables macrophages to metabolize the heme, producing iron for erythropoiesis, in addition to optimizing the iron processing, inhibiting the iron-mediated cytotoxicity. SPI-C was seen to be important to macrophage maintenance in the spleen, as the SPI-C KO mice presented fewer RPMs, likely due to iron toxicity and, consequently, compromised clearance of RBCs and iron recycling.47
The activity of macrophages in iron recycling is mediated by the Hmox-1, which is responsible for the catabolism of the heme to equimolar amounts of iron, biliverdin and carbon monoxide (CO). The Hmox-1 plays a crucial role in the differentiation of erythroblasts, even under stressful conditions, since a reduction in its activity results in an impediment in the maturation of erythroid progenitors.48 Furthermore, the full Hmox-1 KO in mice results in 90% of lethality in the embryonic stage and the survivors present loss of RPMs and fibrosis in the spleen.49 In humans, mutations in the Hmox-1 gene results in hemolysis and asplenia, besides chronic inflammation. Interestingly, it was demonstrated that the CO can induce differentiation of the erythroid cell lineage K562 into mature erythrocytes, showing the role of other factors released by the macrophage in erythroid differentiation.50
Divalent ferrous ion (Fe2+) is the active form present in hemoglobin (and other enzymes), however, it is cytotoxic, as it generates free radicals by the Fenton reaction. Therefore, iron is oxidized to trivalent ferric ion (Fe3+) and either intracellularly stored as ferritin or systemically transported as bound to transferrin molecules (Trf).51 Ferritin was seen to be secreted via exocytosis in the macrophage/erythroblast coculture and subsequently internalized by erythroblasts.52 Free iron, in turn, is exported from the cell by the action of the Fpn1, which is up-regulated within one hour upon erythrophagocytosis.53 Fpn1 is the only iron exporter known, and the Fpn1 KO mice, as well as some Fpn1 polymorphisms in humans, result in iron overload in the spleen and liver.54 After exportation, the iron is then oxidized by the ferroxidase ceruloplasmin and thus, it can be transported into the bloodstream in its ferric form by Trf.55 Since erythroblasts express the Trf receptor (CD71), they can obtain iron bound to the Trf for their hemoglobin synthesis. Recently, it was shown that EBI macrophages also express Trf, suggesting that they can secret Trf in the EBI niche, collaborating in the iron provision for erythroblasts.17Figure 3 illustrates the molecules involved in the clearance of RBCs and iron recycling by macrophages.
Clearance of senescent or damaged RBCs by macrophages. Macrophages from the spleen and liver are responsible for the phagocytosis of aging or injured RBCs through recognition of “eat me” signals and the lack of “self” markers. Macrophages metabolize the ingested hemoglobin and recycle the iron for new erythroblasts in development. The functions of molecules represented in this figure are detailed in the text. Created with BioRender.com.
Whether iron is stored in or transported out of the macrophages depends on the erythropoietic activity or iron needs, which are regulated by hepcidin, a hormone produced by the liver. Hepcidin is produced when plasma iron levels are high, or during infection and inflammation, and induces the Fpn-1 degradation, preventing iron exportation from macrophages to the circulation. In contrast, when iron levels are low or are being used by intense erythropoietic activity, production of hepcidin is reduced and, consequently, Fpn-1 is stabilized and then provides iron exportation from macrophages that can be used in the development of new erythroid cells.56 Therefore, erythrophagocytosis and iron recycling by macrophages is a homeostatic event that can be modulated upon stress situations to adjust the levels of iron, hemoglobin and RBCs to normal. For instance, the toll-like receptor (TLR)-dependent acute inflammation can induce downregulation of the Sirp-α, enhancing the phagocytic capacity of RPMs. In addition, under stress conditions when erythrophagocytosis is enhanced, macrophages from the liver become essential to this task, as monocytes were shown to migrate to the liver and differentiate into iron-recycling macrophages after massive hemolysis.57 Circulating monocytes can also perform erythrophagocytosis, as the RBC marker CD235a was shown inside monocytes from healthy individuals and it was strikingly higher in monocytes from transfused patients, indicating that they can represent a considerable contribution, especially to stress erythropoiesis.58 Treatment with glucocorticoids enhances the ability of monocytes for RBC clearance, up-regulating the expression of the CD163, increasing the hemoglobin (Hb) uptake and shifting the expression of major iron metabolism proteins for an improved heme break-down and iron storage capability.59 Similarly, IL-33 was seen to differentiate monocytes into a subset of tissue-resident macrophages involved in the clearance of senescent erythrocytes and the recycling of their iron content.60
Conclusion and future perspectivesEfficient erythropoiesis and clearance of senescent RBCs is essential for life. Macrophages are central players in the erythrocyte lifespan stages from the beginning to the end, providing factors needed for survival, proliferation and development of erythroid cells, as well as eliminating damaged RBCs from the organism and contributing to the iron detoxication processes during homeostasis and especially upon stress and disorder scenarios. The understanding of macrophage functions and their regulatory mechanisms is currently expanding and there is still much to be uncovered about the signaling pathways involved in the process.
All the factors expressed and secreted by macrophages, their cognate receptors in erythroblasts, and which developmental stages are influenced by them are questions still not completely answered and need more investigation. For instance, depleting models would reveal whether the IL-18 produced by EBI macrophage can change the IL-18R-expressing erythroid cell survival, proliferation or differentiation. Whether EBI macrophages are cells in different stages of maturation/activation or a heterogeneous group of cells is another fundamental question that future single-cell analysis would be able to uncover. Furthermore, revealing the mechanisms by which macrophages regulate the production of the HbF by erythrocytes would be beneficial for sickle cell disease, as high levels of HbF confer improvement in clinical manifestations of the patients. Therefore, clarifying these and other remaining questions is essential to the understanding of how the EBI microenvironment works and to the development of future therapeutic interventions for anemic and inefficient erythropoiesis disorders.
Author contributionsAll authors contributed to the article and approved the submitted version.
The authors thank FAPESP (2019/09704-7 and 2021/05191-5) for the funding support.